
Concept explainers
(a)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
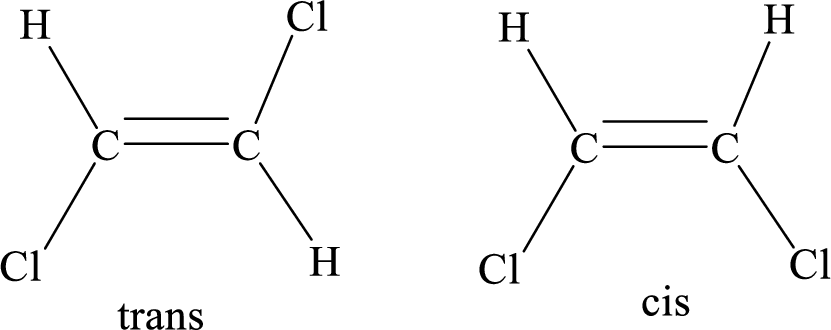
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(b)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
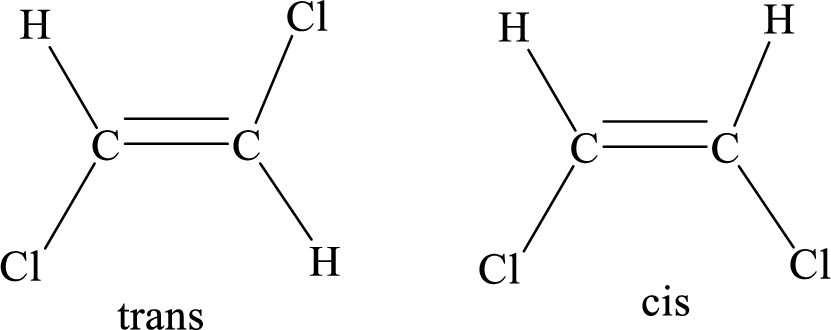
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(c)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
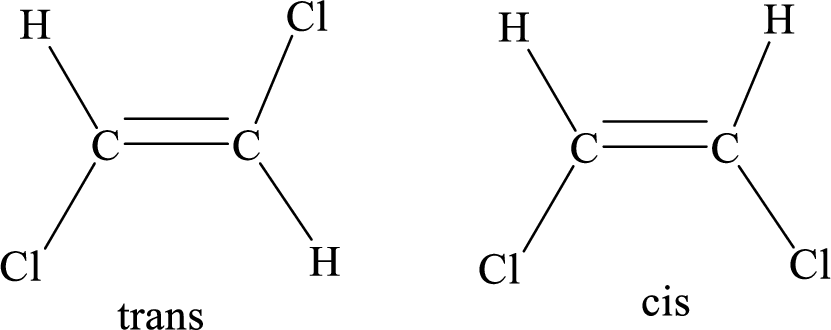
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(d)
Interpretation:
The number and kind of stereoisomers formed when given molecule is treated in presence of
Concept Introduction:
Geometric isomers: Two atoms with the same side location of the double bond are called cis isomers and two atoms with opposite side locations of the double bond are called Trans isomers.
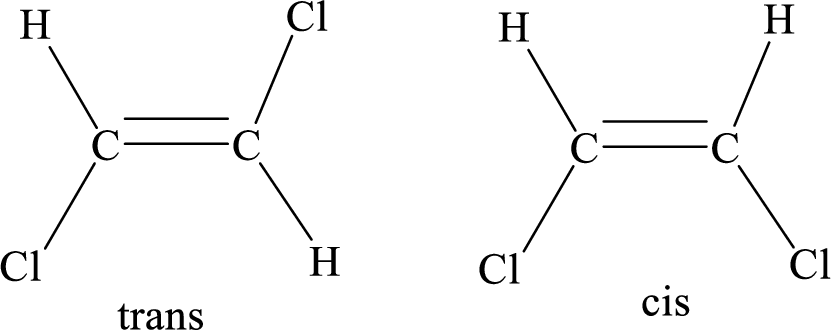
R and S nomenclature: it is used to assign the molecule using CIP (Cahn-Ingold-Prelog) rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Trending nowThis is a popular solution!

Chapter 6 Solutions
Organic Chemistry
- The IUPAC name of the compound is (a) 5-formylhex-2-en-3-one (b) 5-methyl-4-oxohex-2-en-5-al (c) 3-keto-2-methylhex-5-enal (d) 3-keto-2-methylhex-4-enal The correct statement regarding electrophile is (a) electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electrophile (b) electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile (c) electrophile can be either neutral or positively charged species and can form a bond by accepting a pair of electrons from a nucleophile (d) electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile, Which among the given molecules can exhibit tautomerism? Ph Ph I III (a) III only (b) Both I and III (d) Both II and III (c) Both I and II 5) Which of the following biphenyls is optically active? IIarrow_forward6) 25pts. Draw the structure of the major alkene product (or products) formed by treatment of each of the following haloalkanes with sodium ethoxide in ethanol. Assume the mechanism is E2 elimination. t-BuO K t-BUOH Br CH3 Eto Na F ETOH CH2CH3 CI H- Eto Na -CH2CH3 ELOH H- ČH3 Br Eto Na ELOH CH3 CI, H Eto Na CH2CH3 H3C H D ELOHarrow_forwardDraw all of the substitution and elimination products formed from the given alkyl halide with each reagent: (a) CH3OH; (b) KOH. Indicate the stereochemistry around the stereogenic centers present in the products, as well as the mechanism by which each product is formed.arrow_forward
- The IUPAC name of the compound H- is (a) 5-formylhex-2-en-3-one (b) 5-methyl-4-oxohex-2-en-5-al (c) 3-keto-2-methylhex-5-enal (d) 3-keto-2-methylhex-4-enal ( The correct statement regarding electrophile is (a) electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from another electrophile (b) electrophiles are generally neutral species and can form a bond by accepting a pair of electrons from a nucleophile (c) electrophile can be either neutral or ar positively charged species and can form a bond by accepting a pair of electrons from a nucleophile (d) electrophile is a negatively charged species and can form a bond by accepting a pair of electrons from a nucleophile.. Which among the given molecules can exhibit tautomerism? Ph Ph I II III (a) III only (c) Both I and II (b) Both I and III (d) Both II and III 1) Which of the following biphenyls is optically active?arrow_forwardDraw the structure of the organic product formed when 2,3-butanedione reacts with the following reagents. CH3 -C-C-CH3 | (a)H2/Ni (b)l2/NAOH 3Darrow_forwardDraw the constitutional isomer formed when the following alkenes are treated with each set of reagents: [1] H2O, H2SO4; or [2] BH3 followed by H2O2, −OH.arrow_forward
- The reaction of 1-bromopropane and sodium hydroxide in ethanol occurs by an SN2mechanism. What happens to the rate of this reaction under the following conditions?(a) The concentration of NaOH is doubled.(b) The concentrations of both NaOH and 1-bromopropane are doubled.(c) The volume of the solution in which the reaction is carried out is doubled.arrow_forwardCompound X (C4H9Br) reacts by heating with NaOH in H2O to form Y. The compound Y then undergoes acid catalysed hydration by H2SO4 in 180°C to form 2-methyl prop-1-ene. (e) Determine the structure of X and Y. (f) Predict a MAJOR product when compound Y reacts with H2SO4 in 140°C. (g) Draw a structural isomer of X. Name the isomer using IUPAC nomenclature. (h) Describe a chemical test to distinguish between compound Y and 1-butanol.arrow_forwardWhat reaction conditions are needed to convert (R)-2-ethyl-2-methyloxirane to (S)-2- methylbutane-1,2-diol ?arrow_forward
- Draw the structure of two alkenes that would yield 1‑methylcyclohexanol when treated with Hg(OAc)2Hg(OAc)2 in water, then NaBH4NaBH4arrow_forwardDraw the constitutional isomer formed when the attached alkenes are treated with each set of reagents: [1] H2O, H2SO4; or [2] BH3 followed by H2O2, −OH.arrow_forwardHow do you convert:(i) Chlorobenzene to biphenyl(ii) Propene to 1-iodopropane(iii) 2-bromobutane to but-2-enearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





