
Concept explainers
(a)
Interpretation: The conformation which is present in higher concentration when
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The equatorial conformation is present in higher concentration in the given compound.
Explanation of Solution
Given:
The value of
The equilibrium reaction of monosubstituted cyclohexane is shown below.
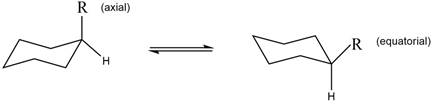
Figure 1
The value of
The equatorial conformation is present in higher concentration in the given compound.
(b)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The
Explanation of Solution
The values of
The both given values are greater than
If the
Therefore, the
The
(c)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The
Explanation of Solution
The values of
The equilibrium constant
Therefore, the
The
(d)
Interpretation: The
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The value of
Explanation of Solution
Given:
The values of
The relationship between
As the value of
The value of
(e)
Interpretation: The explanation corresponding to the relation of size of
Concept introduction: The change in Gibbs free energy is represented by
If the
Answer to Problem 6.37P
The large size of
Explanation of Solution
The value
The large size of
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry - With Access (Custom)
- ∆H° values obtained for a series of similar reactions are one set of experimental data used to determine the relative stability of alkenes. Explain how the following data suggest that cis-but-2-ene is more stable than but-1-ene (Section 12.3A).arrow_forward(a) which a disubstituted cis- alkene? (b) which is the geometric isomer of D? (c) which is a tri-substituted Z- alkene? (d) which is the most stable alkene? (e) which has the highest boiling point? (f) which has the highest MP?arrow_forwardThere are 17 possible alkene isomers with the formula C6H12. Draw structures of the five isomers in which the longest chain has six carbon atoms, and give the name of each. Are any of these isomers chiral? (There are also eight isomers in which the longest chain has five carbon atoms, and four isomers in which the longest chain has four carbon atoms. How many can you find?)arrow_forward
- Rank the conformations of n-butane with reference to its potential energy from the most stable to the least stable. Rank from the most stable to the least stable.To rank items as equivalent, overlap them.arrow_forwardAre the C¬C bonds cyclopropane weaker than those in cyclohexane?arrow_forwardPlease don't provide handwriting solutionarrow_forward
- Draw structures that fit each description and name the functional group in each molecule: (a) two constitutional isomers with molecular formula C5H10O that contain different functional groups; (b) two constitutional isomers with molecular formula C6H10O that contain the same functional group.arrow_forwardDraw the staggered and eclipsed conformations that result from rotation around the C–C bond in CH3–CH2Br.arrow_forwardOn the following energy diagram, identify the energy level of the cyclohexane conformation shown in the box. Energy (kJ/mol) A) I B) II CLU ||| IV Conformations 2arrow_forward
- There are four cis,trans isomers of 2-isopropyl-5-methylcyclohexanol:(a) Using a planar hexagon representation for the cyclohexane ring, draw structural formulas for the four cis,trans isomers.(b) Draw the more stable chair conformation for each of your answers in part (a).(c) Of the four cis,trans isomers, which is most stable? (Hint: If you answered thispart correctly, you picked the isomer found in nature and given the name menthol.)arrow_forwardIndicate the position, axial or equatorial, of the substituents in the more stable chair conformation. CH3 Methyl is [A] OH is [B] Isopropyl is [C] Cl is [D] HO` CIarrow_forward(a) Draw the most stable chair conformers of trans-1,3-diisopropylcyclohexane and trans-1,4-diisopropylcyclohexane. (b) Which is more stable: trans-1,3-diisopropylcyclohexane or trans-1,4-diisopropylcyclohexane?arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning


