
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
∆H° values obtained for a series of similar reactions are one set of experimental data used to determine the relative stability of
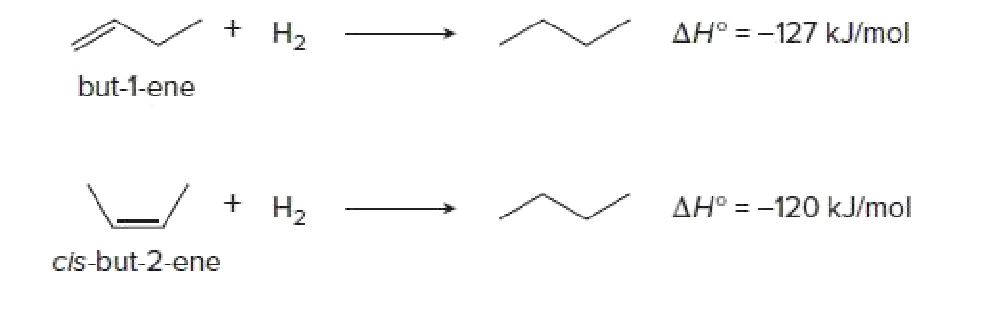
Transcribed Image Text:+ H,
AH° = -127 kJ/mol
but-1-ene
+ H2
AH° = -120 kJ/mol
cis-but-2-ene
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What would be the energy diagram for it?
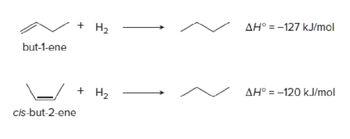
Transcribed Image Text:but-1-ene
+
cis-but-2-ene
H₂₂
+ H₂
AH° = -127 kJ/mol
AH° -120 kJ/mol
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
What would be the energy diagram for it?

Transcribed Image Text:but-1-ene
+
cis-but-2-ene
H₂₂
+ H₂
AH° = -127 kJ/mol
AH° -120 kJ/mol
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- organic chemistry 1) The product of the following reaction is:arrow_forwardDraw the skeletal structure of an isomer of this alkene that does not have any t-bonds in itarrow_forwardDraw a structural formula for the alkene you would use to prepare the alcohol shown by hydroboration/oxidation. CH3 HO CH3 •You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. H3C-CH3 n [ ?arrow_forward
- 1,4-Pentadiene (CH2=CH-CH2-CH=CH2) is a liquid at room temperature and has a density of 0.66 g/mL and molar mass of 68.12 g/mol. In a laboratory experiment, 3.80 mL of this compound was treated with 4.80 mL of conc. H2SO4 (100% w/w; molar mass 98.08 g/mol). Note that the density of conc. H2SO4 is 1.84 g/mL. The resulting sulfate ester was then treated with 1.20 mL of water (molar mass 18.02 g/mol) affording, after work- up, 2,4-pentanediol (molar mass 104.15 g/mol) as the crude product. The crude product was then purified by simple distillation, which yielded 2.00 g of pure product. What is the theoretical yield of 2,4-pentanediol expressed in grams? Show calculations. What is the percentage yield of pure 2,4-pentanediol?arrow_forwardThe hydrogenation of 3-carene can yield two stereoisomeric alkane products. The reaction only yields one of the two products. Please draw the structures and circle the product formed and explain your choice.arrow_forwardDraw the structure of (3E)-3,7-dimethylocta-1,3,6-triene Draw the cis and trans isomers of pent-2-ene and label them.arrow_forward
- Indicate which of the following compounds show geometric (cis-trans) isomerism, draw the isomeric structures, and specify each as Z or E. 2-pentene 1-chloro-2methylbutene 2-bromo-1-chloro-1-hydroxypropene Draw all possible isomers of the following compound 2,5-dimethyl-3-hexene 2-isobutyl-3-methyl-1-hexene Draw the structural isomers of C4H7I. How many can exist as geometric isomers? Identify the picture of each pair of compounds below as identical, structural isomers, geometric isomers or not related.arrow_forwardWhy is a 1, 3 - cis disubstituted cyclohexane more stable than its trans isomer?arrow_forwardWhich of the following is probed by UV- vis spectroscopy? A) Bond stretches B) Bond vibrations C) Electronic transitions D) Molecular rotationarrow_forward
- 1,4-Pentadiene (CH2=CH-CH2-CH=CH2) is a liquid at room temperature and has a density of 0.66 g/mL and molar mass of 68.12 g/mol. In a laboratory experiment, 3.80 mL of this compound was treated with 4.80 mL of conc. H2SO4 (100% w/w; molar mass 98.08 g/mol). Note that the density of conc. H2SO4 is 1.84 g/mL. The resulting sulfate ester was then treated with 1.20 mL of water (molar mass 18.02 g/mol) affording, after work- up, 2,4-pentanediol (molar mass 104.15 g/mol) as the crude product. The crude product was then purified by simple distillation, which yielded 2.00 g of pure product. a. Provide a balanced chemical equation to show the reaction between 1,4-pentadiene and sulfuric acid. Do not use molecular formulas in the chemical equation except for sulfuric acid. b. What reactant is the limiting reagent in this chemical equation? Show calculations to support your answer.arrow_forwardCompounds X and Y have the formula C6H12- Both X and Y react with one molar equivalent of hydrogen in the presence of a palladium catalyst to form 2-methylpentane. The heat of hydrogenation of X is less than that of Y. X and Y react with HBr to form a mixture of the same bromoalkanes, and they both undergo hydroboration/oxidation to give a mixture of the same alcohols. What is the structure of Y? In cases where there is more than one answer, just draw one. n. n [ ]# ChemDoodleⓇ zaarrow_forwardAlkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C–Hg bond to a C–H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY