
Concept explainers
Interpretation: Three figures of barometers in which the centre one is located at the sea level are shown. The barometer that is most likely to reflect the atmospheric pressure in Denver,
Concept introduction: There occurs variation in pressure from one place to another. Different units are used for representing the pressure. The unit
To determine: The barometer that is most likely to reflect the atmospheric pressure in Denver,
Answer to Problem 6.1VP
Solution
The figure (a) is therefore, the correct option.
Explanation of Solution
Explanation
The graph representing the variation of atmospheric pressure at different locations on the surface of the earth that has relation with the mass of the air in column present above that location is shown as,
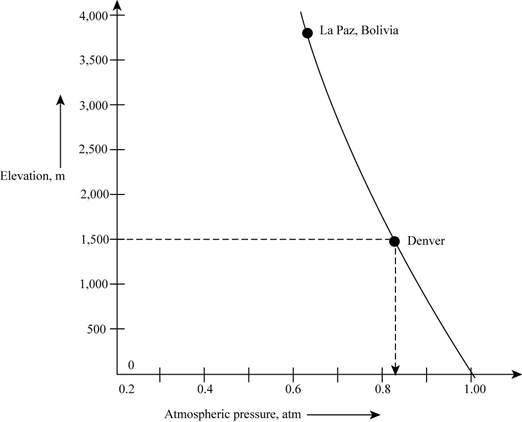
Figure 1
According to the graph, it is observed that with increasing altitude, there occurs a decrease in the mass of the air present above a given area. If mass becomes less it means that the chances of collisions are very less and if the collision will not occur then the force exerted by air will be small.
Therefore, the figure that represents the decrease in column is most likely to reflect the atmospheric pressure in Denver,
Conclusion
The figure that represents the decrease in column is most likely to reflect the atmospheric pressure in Denver,
Want to see more full solutions like this?
Chapter 6 Solutions
Chemistry
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





