
(a)
Interpretation:
Bond-dissociation energy should be identified for the red line bond in the given structure.
Concept introduction:
Bond-dissociation energy (
Bond-dissociation energy is mainly depends on the hybridization of the carbon atom and substitution on the carbon atom
It is also determined by the electronegativity of the molecule and polarization of the molecule to which carbon atom bonded with.
Sigma bond: A covalent bond formation is mainly due to end to end overlap of atomic orbitals.
Pi bond: A covalent bond formation is mainly due to side to side overlap of atomic orbitals
Electronegativity: The ability of an atom to attract electrons towards itself.
Polarization: A partial charge separation between the carbon and an other heteroatom due to its electronegativity difference.
(a)
Answer to Problem 19PP
Answer
The bond dissociation energy of the bonds in red line of the molecule (a) is given below

Carbon - carbon triple bond has more dissociation energy
Carbon - carbon triple bond has more dissociation energy than the carbon – carbon double bond and Carbon – carbon double bond more dissociation than the carbon – carbon single bond.
Explanation of Solution
To find: Bond-dissociation energy of the given compounds.
Draw the given molecule and analyze the nature of C-C bonds present in it.
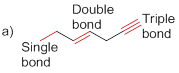
The given molecule is drawn and it has three types of C-C bonds. They are
Carbon – Carbon Single bond has less dissociation energy than carbon – carbon double bond and triple bond. Carbon – carbon triple bond (sp hybridization) has more dissociation energy because it consists of three bonds (two pi bonds and one sigma bond) so it requires more energy to break the bonds than the other carbon- carbon double bonds (one pi bonds and one sigma bond) and carbon – carbon single bond (only one sigma bond).
(b)
Interpretation:
Bond-dissociation energy should be identified for the red line bond in the given structure.
Concept introduction:
Bond-dissociation energy (BDE) is determined from the strength of a single chemical bond.
Bond-dissociation energy is mainly depends on the hybridization of the carbon atom and substitution on the carbon atom
It is also determined by the electronegativity of the molecule and polarization of the molecule to which carbon atom bonded with.
Sigma bond: A covalent bond formation is mainly due to end to end overlap of atomic orbitals.
Pi bond: A covalent bond formation is mainly due to side to side overlap of atomic orbitals
Electronegativity: The ability of an atom to attract electrons towards itself.
Polarization: A partial charge separation between the carbon and an other heteroatom due to its electronegativity difference.
(b)
Answer to Problem 19PP
Answer
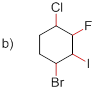
C-F bond has more dissociation energy
Explanation of Solution
To find: Bond-dissociation energy of the given compounds.
Draw the given molecule and analyze the nature of C-C bonds present in it.

The given molecule is drawn and it has four types of C-C bonds. They are
Bond dissociation energy depends on the electronegativity of the molecule and polarization of the molecule. If the molecule has high electronegativity (less polarizability) the dissociation energy of the molecule is high.
The electronegativity order in the halogen series given below
C-F has more Bond dissociation energy in the given molecule since it has more electronegativity than the other halogen atoms.
Want to see more full solutions like this?
Chapter 6 Solutions
ORG. CHEM. TEXT/STUDY GUIDE/EBOOK (LL)
- K Problem 22 of 24 Submit Draw the missing organic structures in the following multistep synthesis at physiological pH (pH = 7.4). Ignore any inorganic byproducts formed. 0 0 ΝΘ BrCH(CO2CH2CH3)2 Select to Draw 1. NaOCH2CH3 2. (CH3)2CHCIarrow_forwardDevise electrochemical cells in which the following reactions could be made to occur. If liquid junctions are necessary, note them in the cell schematic appropriately, but neglect their effects. (a) H2OH + OH¯ (b) 2H2O2 H₂O (c) 2PbSO4 + 2H2O (d) An TMPD PыO₂+ Pb + 4H+ + 20%¯¯ An + TMPD (in acetonitrile, where An and An are anthracene and its anion radical, and TMPD and TMPD are N,N,N',N'-tetramethyl-p-phenylenediamine and its cation radical. Use anthracene potentials for DMF solutions given in Appendix C.3). (e) 2Ce3+ + 2H + BQ 2Ce4+ + H2Q (aqueous, where BQ is p-benzoquinone and H₂Q is p- hydroquinone) (f) Ag +Agl (aqueous) (g) Fe3+ + Fe(CN)6 Fe²+ + Fe(CN) (aqueous)arrow_forwardConsider each of the following electrode-solution interfaces, and write the equation for the elec- trode reaction that occurs first when the potential is moved in (1) a negative direction and (2) a posi- tive direction from the open-circuit potential. Next to each reaction write the approximate potential for the reaction in V vs. SCE (assuming the reaction is reversible). (a) Pt/Cu2+ (0.01 M), Cd2+ (0.01 M), H2SO4(1 M) (b) Pt/Sn2+ (0.01 M), Sn4+ (0.01 M), HCl(1 M) (c) Hg/Cd2+ (0.01 M), Zn2+ (0.01 M), HCl(1 M)arrow_forward
- What are the major products of both of the organic reactions. Please be sure to use wedge and dash bonds to show the stereochemistry of the products if it is needed. Please include the final product as well as a digram/drawing to show the mechanism of the reaction.arrow_forwardK Problem 16 of 24 Submit Draw the starting structure that would yield this product under these conditions. Select to Draw 1. NH4Cl, NaCN 2. HCI, H2O, A NH3 + 0arrow_forwardGive detailed me detailed mechanism Solution with explanation needed. Don't give Ai generated solution. avoid handwritten Solutionarrow_forward
- Show work with explanation needed. don't give Ai generated solutionarrow_forwardK Problem 21 of 24 Submit Draw the missing organic structures in the following multistep synthesis. Show the final product at physiological pH (pH = 7.4). Ignore any inorganic byproducts formed. H 0 NH3 Select to Draw HCN H+, H2O Select to Draw Select to Draw Δarrow_forwardShow work with explanation needed. Don't give Ai generated solution. Give correct solutionarrow_forward
- K Problem 23 of 24 Submit Draw the product of the reaction shown below at physiological pH (pH = 7.4). Ignore inorganic byproducts. S O 1. NH3, 2. HCN 3. H+, H₂O, A Select to Drawarrow_forwardGive detailed mechanism Solution with explanation needed..don't give Ai generated solutionarrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





