
Interpretation: A plot for the variation of partial pressure of
Concept Introduction: The relationship between reactants and products of a reaction in equilibrium with respect to some unit is said to be equilibrium expression. It is the expression that gives ratio between products and reactants. The expression is:
Answer to Problem 111CP
Explanation of Solution
Given:
Initial moles for the ammonia synthesis reaction is:
At
The complete balanced reaction for the formation of ammonia is:
From the balanced reaction it is observed that 1 mole of nitrogen gas reacts with 3 moles of hydrogen gas to produce 2 moles of ammonia gas.
- For the total pressure of 1.0 atm:
Let the partial pressure of nitrogen gas be x atm so, the partial pressure of hydrogen gas will be 3x atm. Thus,
Solving for x:
So, the partial pressure of nitrogen gas is
The ICE table for the reaction will be set up as:
The expression for the equilibrium constant is:
Substituting the values:
Assuming the change be small so,
Solving for x:
The correct value of x is calculated by substituting the assumed x value as:
The value of x is used and this process is repeated until the value of x does not vary:
Since, the value x has not changed so, this value of x used. Thus, the partial pressure of ammonia is:
- For the total pressure of 10.0 atm:
The ICE table for the reaction will be set up as:
The expression for the equilibrium constant is:
Substituting the values:
Assuming the change be small so,
Solving for x:
The correct value of x is calculated by substituting the assumed x value as:
The value of x is used and this process is repeated until the value of x does not vary. So, the calculated values of x are:
Since, the value x has not changed so, this value of x used. Thus, the partial pressure of ammonia is:
- For the total pressure of 100.0 atm:
The ICE table for the reaction will be set up as:
The expression for the equilibrium constant is:
Substituting the values:
The value of x is calculated using successive approximations as:
Solving for x:
The correct value of x is calculated by substituting the assumed x value as:
The value of x is used and this process is repeated until the value of x does not vary. So, the calculated values of x are:
Since, the value x has 16 is repeated so, this value of x used. Thus, the partial pressure of ammonia is:
- For the total pressure of 1000.0 atm:
The ICE table for the reaction will be set up as:
The expression for the equilibrium constant is:
Substituting the values:
The value of x is calculated using successive approximations as:
Solving for x:
The correct value of x is calculated by substituting the assumed x value as:
The value of x is used and this process is repeated until the value of x does not vary. So, the calculated values of x are:
Since, according to significant digits, the value x has 220 is repeated so, this value of x used. Thus, the partial pressure of ammonia is:
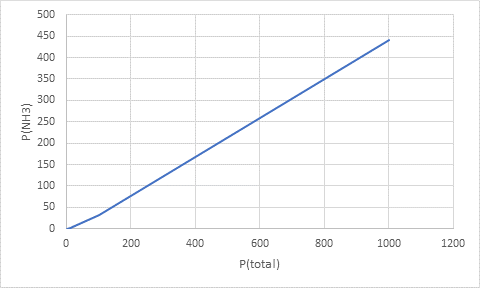
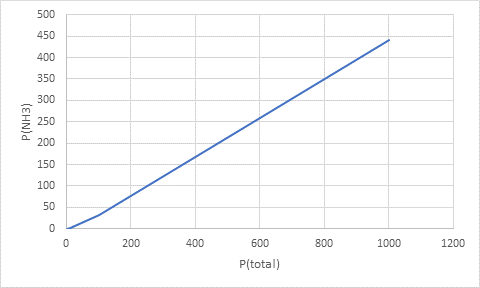
Thus, the values of partial pressure of ammonia at total pressures are:
| total pressure (atm) | partial pressure of ammonia(atm) |
| 1.0 | 0.024 |
| 10.0 | 1.4 |
| 100.0 | 32 |
| 1000.0 | 440 |
Plotting the data as:
Want to see more full solutions like this?
Chapter 6 Solutions
Chemical Principles
- For the reaction N2(g)+3H2(g)2NH3(g) show that Kc = Kp(RT)2 Do not use the formula Kp = Kc(RT)5n given in the text. Start from the fact that Pi = [i]RT, where Pi is the partial pressure of substance i and [i] is its molar concentration. Substitute into Kc.arrow_forwardConsider 0.200 mol phosphorus pentachloride sealed in a 2.0-L container at 620 K. The equilibrium constant, Kc, is 0.60 for PCl5(g) PCl3(g) + Cl2(g) Calculate the concentrations of all species after equilibrium has been reached.arrow_forwardWrite an equation for an equilibrium system that would lead to the following expressions (ac) for K. (a) K=(Pco)2 (PH2)5(PC2H6)(PH2O)2 (b) K=(PNH3)4 (PO2)5(PNO)4 (PH2O)6 (c) K=[ ClO3 ]2 [ Mn2+ ]2(Pcl2)[ MNO4 ]2 [ H+ ]4 ; liquid water is a productarrow_forward
- For the reactionH2(g)+I2(g)2HI(g), consider two possibilities: (a) you mix 0.5 mole of each reactant. allow the system to come to equilibrium, and then add another mole of H2 and allow the system to reach equilibrium again. or (b) you mix 1.5 moles of H2 and 0.5 mole of I2 and allow the system to reach equilibrium. Will the final equilibrium mixture be different for the two procedures? Explain.arrow_forwardShow that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation KI(aq)+I2(aq)KI3(aq) give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3-.arrow_forwardThe equilibrium constant Kc, for the reaction 2 NOCI(g) 2 NO(g) + Cl2(g) is 3.9 103 at 300 C. A mixture contains the gases at the following concentrations: [NOCl] = 5.0 103 mol/L, [NO] = 2.5 103 mol/L, and [Cl2] = 2.0 103 mol/L. Is the reaction at equilibrium at 300 C? If not, in which direction does the reaction proceed to come to equilibrium?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





