
Essential Organic Chemistry, Global Edition
3rd Edition
ISBN: 9781292089034
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.9, Problem 19P
Draw a reaction coordinate diagram for the following reaction in which C is the most stable and B the least stable of the three species and the transition state going from A to B is more stable than the transition state going from B to C:
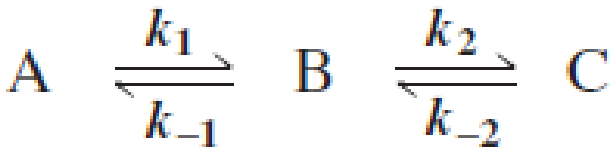
- a. How many intermediates are there?
- b. How many transition states are there?
- c. Which step has the greater rate constant in the forward direction?
- d. Which step has the greater rate constant in the reverse direction?
- e. Of the four steps, which has the greatest rate constant?
- f. Which is the rate-determining step in the forward direction?
- g. Which is the rate-determining step in the reverse direction?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify SN1 transition state properties.
Choose one or more:
O A. There are two transition states in an SN1 reaction.
B. There is one transition state in an SN1 reaction.
O C. The transition state(s) is/are higher in energy than the reactants.
O D. The transition state(s) is/are lower in energy than the reactants.
O E. The transition state(s) is/are higher in energy than the intermediate(s).
F. The transition state(s) is/are lower in energy than the intermediate(s).
O G. The transition state(s) have the same energy as the intermediate(s).
O H. For a mechanism that contains n total steps, there must be ntotal transition states.
O O
Draw a reaction coordinate diagram for the following reaction in which C is the most stable and B the least stable of the three species and the transition state going from A to B is more stable than the transition state going from B to C:a.How many intermediates are there? b. How many transition states are there? c. Which step has the greater rate constant in the forward direction? d. Which step has the greater rate constant in the reverse direction? e. Of the four steps, which has the greatest rate constant? f. Which is the rate-determining step in the forward direction? g. Which is the rate-determining step in the reverse direction?
Consider the following energy diagram.
a.How many steps are involved in this reaction?
b. Label ΔHo and Ea for each step, and label ΔHooverall.
c.Label each transition state.
d.Which point on the graph corresponds to a reactive intermediate?
e.Which step is rate-determining?
f. Is the overall reaction endothermic or exothermic?
Chapter 5 Solutions
Essential Organic Chemistry, Global Edition
Ch. 5.1 - Prob. 1PCh. 5.1 - Draw the structure for each of the following: a....Ch. 5.1 - What is each compounds systematic name?Ch. 5.3 - Prob. 4PCh. 5.3 - Prob. 5PCh. 5.3 - Prob. 7PCh. 5.4 - a. For which reaction in each set below will S be...Ch. 5.6 - Prob. 9PCh. 5.6 - How many different alkenes can be hydrogenated to...Ch. 5.6 - The same alkane is obtained from the catalytic...
Ch. 5.6 - Prob. 12PCh. 5.6 - Rank the following compounds in order from most...Ch. 5.7 - The rate constant for a reaction can be increased...Ch. 5.7 - Prob. 15PCh. 5.7 - Prob. 16PCh. 5.9 - Draw a reaction coordinate diagram for a two-step...Ch. 5.9 - Prob. 18PCh. 5.9 - Draw a reaction coordinate diagram for the...Ch. 5.10 - Which of the following parameters would be...Ch. 5 - What is each compounds systematic name?Ch. 5 - Draw the structure of a hydrocarbon that has six...Ch. 5 - Which of the following compounds is the most...Ch. 5 - Prob. 24PCh. 5 - Prob. 25PCh. 5 - Prob. 26PCh. 5 - Prob. 27PCh. 5 - Name the following:Ch. 5 - Prob. 29PCh. 5 - Prob. 30PCh. 5 - Prob. 31PCh. 5 - Prob. 32PCh. 5 - Prob. 33PCh. 5 - Prob. 34PCh. 5 - Prob. 35PCh. 5 - Name each of the following:Ch. 5 - Prob. 38PCh. 5 - Given that the twist-boat conformer of cyclohexane...Ch. 5 - a. The G for conversion of axial fluorocyclohexane...Ch. 5 - Prob. 1PCh. 5 - Prob. 2PCh. 5 - Prob. 3PCh. 5 - Prob. 4PCh. 5 - Prob. 5PCh. 5 - Prob. 6PCh. 5 - Prob. 7PCh. 5 - Prob. 8PCh. 5 - Prob. 9PCh. 5 - Prob. 10P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following two reactions H+Cl2HCl+Cl H+Br2HBr+Br the first reaction has a lower value of A than the second reaction. What can one say about the relative properties of the intermediates HCl2 and HBr2, just from the relative values of A?arrow_forwardThe rate of a certain reaction doubles for every 10 C rise in temperature. (a) How much faster does the reaction proceed at 45 C than at 25 C? (b) How much faster does the reaction proceed at 95 C than at 25 C?arrow_forwardThe following reactions are proposed. Make a rough estimate of the rate of each one rapid, slow, wont react. Explain each answer. a.H2O(l)+H+(aq)H3O+(aq) b.H3O+(aq)+H+(aq)H4O2+(aq) c.3H2(g)+N2(g)2NH3(g) d.Ba2+(aq)+SO42-(aq)BaSO4(s)arrow_forward
- The reaction for the formation of product X:A + B --> C + D (slow)B + D --> X (fast)The intermediate in the reaction is...a. Xb. Cc. Ad. Be. Darrow_forwardReaction A and reaction B have identical frequency factors, but reaction B has a higher activation energy than reaction A. Which reaction has a faster rate at room temperature? A. Reaction A B. Reaction Barrow_forwardThe rate of a reaction is found to increase nine-fold when the concentration of one reactant is tripled. The order of the reaction with respect to this reactant is a. first.b. second.c. one-quarter. d. one-half. e. third.arrow_forward
- Which explains increasing rate of reaction with increasing temperature? Frequency of collisions A. B. C. D. Particles with E> E, same more same more same greater greater samearrow_forwardThe kinetics of a reaction are observed to be third-order. The least likely mechanism a. involves three molecules reacting together in a single step. b. involves a fast step followed by a slow step. c. involves more than one intermediate. d. involves a single intermediate.arrow_forwarda. What are the intermediates? b. What is the overall reaction?arrow_forward
- Proposed mechanism for a reaction is O3 + NO2 -> NO3 + O2 slow NO3 + NO2 -> N2O5 fast What are the intermediates in the proposed mechanism? Select one: a.O3 b.NO2 c.N2O5 d.NO3arrow_forwardDetermine the number of transition states and intermediates if you consider the energy diagram of the multi-step reaction below? ÇH3 ÇH3 ÇH3 ÇH3 OH H20 step 2 OH* +cr step 3 -CI step 1 + Cr A B D A. 3 transition states and 3 intermediates B. 2 transition states and 2 intermediates C. 3 transition states and 2 intermediates D. 2 transition states and 3 intermediates E. 2 transition states and 1 intermediatearrow_forward7. Which of the following statements is correct? A. The AG° of a reaction depends on the mechanism (i.e., the path). B. The AG* of a reaction depends on the mechanism. C. The AG° of reactions may be changed by adding a catalyst. D. The bigger the AG°, the faster the reaction. E. The bigger the AG°, the slower the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License