
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5.10A, Problem 5.16P
For each sot of examples, make a model of the first structure, and indicate the relationship of each of the other structures to the first structure Examples of relationships same compound, enantiomer structural isomer.
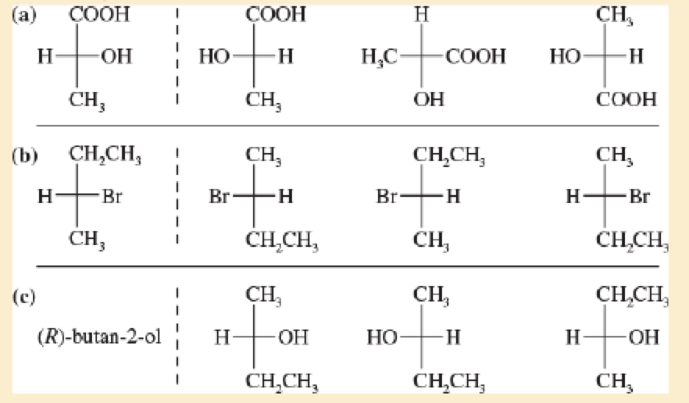
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Some of the following examples can show geometric isomerism, and some cannot. Forthe ones that can, draw all the geometric isomers, and assign complete names using theE-Z system. cyclohexene cyclodecene
Calculate strain for the conformer picture below. Using strain energy increments from the table.
1) Are the molecules A and B... conformational isomers? Diastereomers? Enantiomers? Position isomers? Non-related?
2) What about the molecules B and C?
3) What about the molecules C and D?
Chapter 5 Solutions
Organic Chemistry (9th Edition)
Ch. 5.2 - Determine whether the following objects are chiral...Ch. 5.2A - Prob. 5.2PCh. 5.2B - Prob. 5.3PCh. 5.2B - Prob. 5.4PCh. 5.2C - Prob. 5.5PCh. 5.3 - Prob. 5.6PCh. 5.3 - Prob. 5.7PCh. 5.4D - Prob. 5.8PCh. 5.4D - Prob. 5.9PCh. 5.4D - Prob. 5.10P
Ch. 5.5 - Prob. 5.11PCh. 5.7 - When optically pure (R)-2-bromobutane is heated...Ch. 5.7 - Prob. 5.13PCh. 5.8 - Prob. 5.14PCh. 5.9B - Draw three-dimensional representations of the...Ch. 5.10A - For each sot of examples, make a model of the...Ch. 5.10A - Draw a Fischer projection for each compound....Ch. 5.10B - Prob. 5.18PCh. 5.10C - For each Fischer projection, label each asymmetric...Ch. 5.11C - Prob. 5.20PCh. 5.13 - Prob. 5.21PCh. 5.13 - Prob. 5.22PCh. 5.15 - Prob. 5.23PCh. 5.16A - Prob. 5.24PCh. 5 - The following four structures are naturally...Ch. 5 - For each structure, 1. star () any asymmetric...Ch. 5 - Prob. 5.27SPCh. 5 - Prob. 5.28SPCh. 5 - Prob. 5.29SPCh. 5 - Prob. 5.30SPCh. 5 - Prob. 5.31SPCh. 5 - Prob. 5.32SPCh. 5 - Prob. 5.33SPCh. 5 - Prob. 5.34SPCh. 5 - For each structure, 1. draw all the stereoisomers....Ch. 5 - Prob. 5.36SPCh. 5 - Prob. 5.37SPCh. 5 - 3,4-Dimethylpent-1-ene has the formula...Ch. 5 - A graduate student was studying enzymatic...Ch. 5 - Prob. 5.40SPCh. 5 - Prob. 5.41SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
141. Design a device that uses as electrochemical cell to determine amount of
in a sample water Describe, in...
Chemistry: Structure and Properties (2nd Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
The chapter sections to review are shown in parentheses at the end of each problem. A "chemical-free” shampoo i...
Basic Chemistry
Describe the orbitals used in bonding and the bond angles in the following compounds: a. CH3O b. CO2 c. H2CO d....
Organic Chemistry (8th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- feces, gallston 3a pue Using the structure and image below, determine the conformation of each of the designated substituents. (Use the carbon atom numbering from the image on the left.) HG. HO3 bail & stick labels OH at C3: H, at Cl: Hydrogen at CB: Submit Answer Retry Entire Group 6 more group attempts remainingarrow_forwardGive typed solution not writtenarrow_forwardSubject :- Chemistryarrow_forward
- Give typing answer with explanation and conclusionarrow_forwardFor each of tthe following structural formulas, provide the indicated number of resonance structures. Use curved arrows to show the electron movement between each structure, use double headed arrow to separate the structures, and enclose all of them in a single set of bracketsarrow_forwardTwo compounds are considered as isomers if they have the…arrow_forward
- Can you give me detail about the chiral carbon step by step with an examples, please?arrow_forwardClassify the pairs of molecules as not the same molecule, structural isomers, diastereomers, enantiomers or identical. Circle any molecules that are not achiral (a) (b) (c) Br... H CI H H Br H H3C Br H. CI Br H Br CI I I Br" "H H Br H CH3 Cr Brarrow_forwardIn the structure provided choose the option YES or NO to indicate if the carbon atoms are chiral. H₂N A !!! B ...!!!! OH C CIarrow_forward
- Fill in the blank. Constitutional Isomer? Conformational Isomer? Enantiomer? Diastereomer?arrow_forwardMatch the pair of compounds with the type of isomerism. Choices:a. positional isomerb. cis, trans configurationc. diastereomerd. enantiomere. functional isomerf. skeletal isomerg. E.Z configurationarrow_forwardCan you help me with the explanation of the alkane, alkene, alkyne, aromatic and all these terms give me examples too?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License