
Concept explainers
(a)
Interpretation:
The balanced chemical equation for decomposition of
Concept Introduction:
In a balanced chemical equation, all the constituents present in the reaction have equal number of atoms on both side of the reaction arrow.
Answer to Problem 89P
Explanation of Solution
Decommission of solid Ammonium Nitrate generates gaseous dinitrogen oxide along with the water vapour.
The balanced chemical equation of the reaction taking place is as depicted below:
From the above balanced reaction, for each mole of NH4 NO3 produces one mole of di-nitrogen oxide and 2 moles of water.
(b)
Interpretation:
The partial pressure of
Concept Introduction:
To calculate the partial pressure of
Where,
Answer to Problem 89P
Partial pressure of
Explanation of Solution
Calculate the number of moles of
Therefore, number of moles of
Calculate the number of moles of water is as follows:
Therefore, number of moles of water is 0.0624 mol H2 O.
From the ideal equation we have.
To calculate the partial pressure of
Therefore, the partial pressure of
Volume of tank v = 1.75 L.
Number of moles of water n = 0.0624 moles.
The temperature t = 503 K.
To calculate the partial pressure of water, substitute all the known value in the equation.
Therefore, partial pressure of water is 1.47 atm.
(c)
Interpretation:
The total gas pressure present in the flask at 2300 C should be determined.
Concept Introduction:
Dalton's law of partial pressure is state that the total pressure of a mixture gases is sum of the pressures that every gas would exert if it were present alone.
Answer to Problem 89P
Total pressure present in flask is 2.21 atm.
Explanation of Solution
In the provided reaction the gases molecules are
Therefore, total pressure present in flask is 2.21 atm.
(d)
Interpretation:
The three equivalent resonance structure for
Concept Introduction:
The resonance structures show the arrangement of electrons and bonds in a molecule. The lone pair present on atom can show delocalization with pi electrons of double or triple bonds resulting formation of resonance structures. The position of atoms remains the same only position of bonds changes.
Answer to Problem 89P
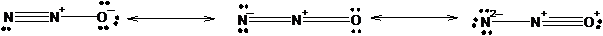
Explanation of Solution
The resonance structures of N2 O is shown in the following diagram.
Since, nitrogen has 3 valence electrons it can form three covalent bonds with other atoms. There are two valence electrons in oxygen thus, it can form one double or two single bonds with other atoms. Being more electronegative in nature, oxygen atom will be placed at the terminal position. Thus, there will be one double bond between two nitrogen atom and one double bond between nitrogen and oxygen atom resulting negative charge on one nitrogen atom and positive charge on other nitrogen atom.
The negative charge can delocalize with pi electrons of double bond resulting two resonance forms.
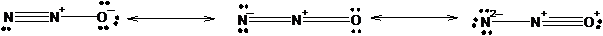
Want to see more full solutions like this?
Chapter 5 Solutions
Introduction to General, Organic and Biochemistry
- JON Determine the bund energy for UCI (in kJ/mol Hcl) using me balanced chemical equation and bund energies listed? का (My (9) +36/2(g)-(((3(g) + 3(g) A Hryn = -330. KJ bond energy и-н 432 bond bond C-1413 C=C 839 N-H 391 C=O 1010 S-H 363 б-н 467 02 498 N-N 160 N=N 243 418 C-C 341 C-0 358 C=C C-C 339 N-Br 243 Br-Br C-Br 274 193 614 (-1 214||(=olin (02) 799 C=N 615 AALarrow_forwardDetermine the bond energy for HCI ( in kJ/mol HCI) using he balanced cremiculequecticnand bund energles listed? also c double bond to N is 615, read numbets carefully please!!!! Determine the bund energy for UCI (in kJ/mol cl) using me balanced chemical equation and bund energies listed? 51 (My (9) +312(g)-73(g) + 3(g) =-330. KJ спод bond energy Hryn H-H bond band 432 C-1 413 C=C 839 NH 391 C=O 1010 S-1 343 6-H 02 498 N-N 160 467 N=N C-C 341 CL- 243 418 339 N-Br 243 C-O 358 Br-Br C=C C-Br 274 193 614 (-1 216 (=olin (02) 799 C=N 618arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





