
Concept explainers
Artemisinin and mefloquine are widely used antimalarial drugs. A ball-and-stick model of artemisinin appears on the cover of this text.
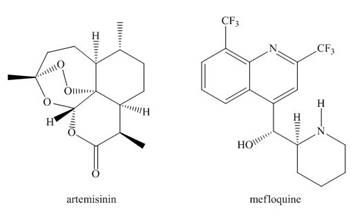
a. Locate the stereogenic centers in both drugs.
b. Label each stereogenic center in mefloquine as
c. What is the maximum number of stereoisomers possible for artemisinin?
d. How are the
e. Can two molecules of artemisinin intermolecularly hydrogen bond to each other?
f. What product is formed when mefloquine is treated with
Trending nowThis is a popular solution!

Chapter 5 Solutions
Organic Chemistry (6th Edition)
Additional Science Textbook Solutions
Organic Chemistry
Principles of General, Organic, Biological Chemistry
Chemistry: The Central Science (14th Edition)
Chemistry
Chemistry by OpenStax (2015-05-04)
Thermodynamics, Statistical Thermodynamics, & Kinetics
- .Draw the strongest IMF that can form between each structure below and a water molecule. Draw a stereoisomer of each molecule below. a. HOarrow_forwardHow many stereocenters are found in the compound shown above? a. 1 b. 2 c. 3 d. 5arrow_forwardThapsigargin is a natural product with promising anticancer properties.a.At which sites can thapsigargin hydrogen bond to another molecule like itself? b.At which sites can thapsigargin hydrogen bond to water? c.How many sp2 hybridized C's are present? d.How many sp3 hybridized 3° C's are present?arrow_forward
- 15. Naming Stereoisomers with Two Chiral Carbons Using the RS System The (RR) isomer of methyphenidate (Ritalin) is used to treat attention deficit hyperactivity disorder (ADHD). The (S.S) isomer is an antidepressant. Identify the two chiral carbons in the structure below. Is this the (R.R) or the (S.S) isomer? Draw the other isomer. HN- H. ..arrow_forward2. How many stereoisomers exist for the following molecule? OH A. 2 B. 3 C. 4 D. 6 E 8 23 The following structures represent CH Br CH,Br CH,Br CH,Br A. enantiomers B. diastereomers C. constitutional isomers D. conformers E. none of thesearrow_forwardHow many stereogenic carbons are there in zuranolone? A. 2 B. 4 C. 6 D. 8 zurandone CNarrow_forward
- 2. What is the relationship between these two molecules? o a. Identical b. Constitutional isomers c. Enantiomers d. Diastereomersarrow_forward1. Identify the relationship between the following two structures.a. Identicalb. neitherc. diastereomers2. Identify the relationship between the following two structures.a. enantiomersb. diastereomersarrow_forwardSaquinavir (trade name Invirase) is a protease inhibitor, used to treat HIV (human immunodeficiency virus). a.Locate all stereogenic centers in saquinavir, and label each stereogenic center as R or S. b.Draw the enantiomer of saquinavir. c.Draw a diastereomer of saquinavir. d.Draw a constitutional isomer that contains at least one different functional group.arrow_forward
- Glucose is a simple sugar with five substituents bonded to a sixmembered ring.a.Using a chair representation, draw the most stable arrangement of these substituents on the six-membered ring. b.Convert this representation to one that uses a hexagon with wedges and dashed wedges. c.Draw a constitutional isomer of glucose. d.Draw a stereoisomer that has an axial OH group on one carbon.arrow_forwardMatch the pairs of compounds with the type of isomerism a. functional isomer b. skeletal isomer c. positional isomer d. cis, trans configuration e. diastereomer f. e,z, configuration g. enantiomerarrow_forwardDrawn are four isomeric dimethylcyclopropanes. a.How are the compounds in each pair related (enantiomers, diastereomers, constitutional isomers): A and B; A and C; B and C; C and D? b.Label each compound as chiral or achiral. c.Which compounds alone would be optically active? d.Which compounds have a plane of symmetry? e.How do the boiling points of the compounds in each pair compare: A and B; B and C; C and D? f.Which of the compounds are meso compounds? g.Would an equal mixture of compounds C and D be optically active? What about an equal mixture of B and C?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





