
Introduction to General, Organic and Biochemistry
11th Edition
ISBN: 9781285869759
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Glucose is a simple sugar with five substituents bonded to a sixmembered ring.
a.Using a chair representation, draw the most stable arrangement of these substituents on the six-membered ring.
b.Convert this representation to one that uses a hexagon with wedges and dashed wedges.
c.Draw a constitutional isomer of glucose.
d.Draw a stereoisomer that has an axial OH group on one carbon.
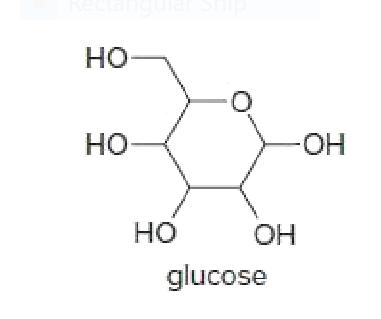
Transcribed Image Text:но
Но
-HO-
OH
glucose
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why specific product formsarrow_forward1. What are the products of a combustion reaction? 2. Describe how the formation of these products is confirmed in the video. 3. Write the balanced equations for the combustion of the following hydrocarbons: a) Propane b) Cyclopentane c) 2-methyl-2-butene heludearrow_forwardOH O OH DCC pyridinearrow_forward
- on Name the product of the following reaction. H + H₂ A/ 신 ‒‒‒‒‒ ->arrow_forward→> CH3CH₂OH HCl n + 0 OH CH CH OH 3 HCIarrow_forwardClassify these sugars by using a name that indicates both the number of carbons and the main functional group present in each. CHO Но a) H- OH H- OH ČH2OH CH2OH HO b) НО H- OH ČH2OHarrow_forward
- 1. Simple sugars are defined as polyhydroxy aldehydes/ketones. All carbons except one have an OH group. The remaining carbon has a double bond to oxygen, making it an aldehyde or ketone. For example, the Fisher projection of glucose looks like this: C H- H- -ОН НО -ОН -HO- CH2OH Because a simple sugar has both a carbonyl carbon and built-in nucleophiles, a reaction can occur within the molecule itself to create the hemi-acetal form of the sugar. Propose a mechanism for this reaction when it occurs in an aqueous solution. C -- CH2OH он в CH2OH HO- H H НО H. ОН Н and ОН Н HO- он Он а OH H H- ОН OH H ОН ČH2OHarrow_forwardIdentify the products of the two reactions of glucose. COOH HOH Н НО H Н HOH Н- -OH CH₂OH Н НО CH2OH -OH H- H -Н -OH OH CH₂OH Cu2+/OH- CHO O НО н H OH H-+ -OH CH₂OH Н но- Н- Н- CHO HOH но- Н- -Н -ОН H-OH CHO CHO -ОН -Н -ОН -ОН CH2OH Answer Bank 0 CH3 H— НО -OH -H H-OH H-OH CH₂OH H₂/Pt CHO H-OH HO-H H -ОН H OH COOH CH₂OH =0 HO-H H- Н -ОН -OH CH₂OHarrow_forwardwhat is the name of CH3COO-arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning