
Concept explainers
Solids and liquids are better conductors than gases.
Explanation of Solution
Introduction:
In heat
In solids, heat conduction is better. In cases of gases, it is worst.
In the case of solids, particles are tightly bonded. So, their positions are almost fixed with respect to every other particle as shown in Figure 1. The force between the two particles is robust, which makes the heat transfer process (by a collision) more efficient.
In the case of liquids, the particles are not tightly packed. So, they can move around within it. The force between two liquid particles is not as strong as solids but better than gases. Thus, liquids are usually poor conductors in comparison to solids.
In the case of gases, the particles are far apart from each other. So, the energy transfer due to collision is very inefficient in this case. Thus, gases, like air, are very poor conductors than solids and liquids. In gases, heat transfer takes place by
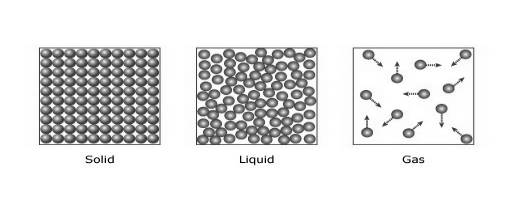
Figure 1
Chapter 5 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
Sears And Zemansky's University Physics With Modern Physics
The Cosmic Perspective Fundamentals (2nd Edition)
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
Applied Physics (11th Edition)
Introduction to Electrodynamics
Physics for Scientists and Engineers: A Strategic Approach, Vol. 1 (Chs 1-21) (4th Edition)
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





