
Concept explainers
(a)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of
Example:
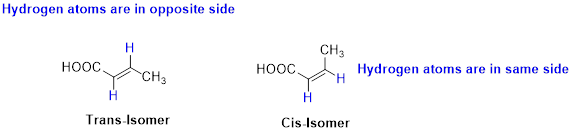
(b)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

(c)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

(d)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

(e)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

(f)
Interpretation:
E or Z configuration has to be identified for the given compounds.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Essential Organic Chemistry, Global Edition
- Figure 7-3 애arrow_forwardHow many of the double bonds in the molecules below have an E-configuration? Is 3 4 1 2 Br Brarrow_forwardWhich of the following is the correct wedge and dash structure for the following Newman projection? HØ CH3 CI Et OI ον O III OIV CH3 CH3 O II 3 CI = It wou ||| IV Varrow_forward
- How many delocalized π electrons are in the following molecule?arrow_forwardChloramphenicol is a broad-spectrum antibiotic that is particularly useful against typhoid fever. What is the configuration of each asymmetric carbon in chloramphenicol? HỌ H c-c-CH,OH NHCCHCI, NO2 chloramphenicolarrow_forwardWhat kind of orbital overlap forms the delocalized p system in a benzene ring? S sp sp3arrow_forward
