
Concept explainers
(a)
Interpretation:
R or S and E or Z configuration has to be assigned for the given compound.
Concept introduction:
The carbon-carbon double bond containing organic compounds are is known as alkenes or olefins, they are one of the class of hydrocarbon.
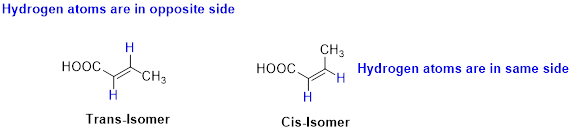
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:
(b)
Interpretation:
The systematic name should be given for the compound.
Concept introduction:
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(c)
Interpretation:
R or S and E or Z configuration has to be assigned for the given compound.
Concept introduction:
Cis–trans isomerism (or) geometric isomerism or configurational isomerism:
The two similar groups (or higher priority groups) are in same side in double bond of alkenes is called as cis isomer (or Z-isomer). Two similar groups (or higher priority groups) are opposite side in double bond of alkenes is called as trans isomer (or E-isomer).
Example:

(c)
Interpretation:
The systematic name should be given for the compound.
Concept introduction:
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Essential Organic Chemistry, Global Edition
- Propose two molecular formulas for each molecular ion: (a) 102; (b) 98; (c) 119; (d) 74.arrow_forward(30) Take a look at the two multi-substituted benzene compounds below. Trinitrotoluene (TNT) has its NO, groups on carbons 2, 4, and 6. Which of the two fits this numerical scheme? Hint: Toluene is a special name for benzene whose primary functional group is a methyl group substituted in place of one of its hydrogens. CH3 CH3 O,N. ZON O,N NO2 NO2 NO2arrow_forwardUsing the information provided below, deduce the identity of the compound IV. What is the IUPAC name of compound IV? ... II AICI3 V 1) O3 [H+], CH3NH2 NABH;CN (C10H12) 2) DMS (C9H100) (C10H15N) -H20 1) EtMgBr 2) H20 IV (C11H160) (A 2-phenyl-2-propanone B 3-phenyl-3-pentanol c) none of these D 1-phenyl-3-pentanol E 1-phenyl-1-propanonearrow_forward
- 7:21 PM Thu Jul 7 2req 2req ts 2req pts 2req 1 pts 2req [Review Topics] Provide an IUPAC name for the structure shown. ball & stick-labels (Do not use stereochemical terms in you name.) Submit Answer Retry Entire Group 9 more group attempts remaining [References] 29%arrow_forward6. The mendelic acid "chemical face peel" is a relatively new innovation for the rejuvenation of problem skin. In addition to removing dead skin cells, its antibacterial properties help acne-prone skin. The technique has also been touted as a way to improve hyperpigmentation due to post acne scarring. Although the compound was first derived from bitter almonds, mendelic acid is generally now synthesized in the laboratory. To formulate one of these "face peel" emulsions, you are asked to prepare as much pH = 3.799 buffer solution as you can from 100.00 mL of 0.5000 M mendelic acid (HMend). Since the use of mendelic acid is relatively new, the conjugate base mendolate (Mend) cannot be purchased. Fortunately, you have plenty of 0.4087 M NaOH. Ka = 3.894x10-4 for mendelic acid. How would you prepare the buffer solution? The following questions will guide you to find the final answer. What is the moles ratio between the equilibrium molarity of mendelic acid (MA) and mendolate? Please…arrow_forward6. Which of the following does NOT depict resonance structures? (1) CH3-0-CH-CH3 CH3 0 CH CH3 (2) CH3 NO CH3-NO :O: (3) CH3 CH3- (4)arrow_forward
- 6. The mendelic acid "chemical face peel" is a relatively new innovation for the rejuvenation of problem skin. In addition to removing dead skin cells, its antibacterial properties help acne-prone skin. The technique has also been touted as a way to improve hyperpigmentation due to post acne scarring. Although the compound was first derived from bitter almonds, mendelic acid is generally now synthesized in the laboratory. To formulate one of these "face peel" emulsions, you are asked to prepare as much pH = 3.543 buffer solution as you can from 100.00 mL of 0.5000 M mendelic acid (HMend). Since the use of mendelic acid is relatively new, the conjugate base mendolate (Mend) cannot be purchased. Fortunately, you have plenty of 0.4018 M NaOH. Ka = 3.894x 10-4 for mendelic acid. How would you prepare the buffer solution? The following questions will guide you to find the final answer. A B. C. What is the moles ratio between the equilibrium molarity of mendelic acid (MA) and mendolate?…arrow_forward30). Pleuromutilin (shown below on the left) is an interesting organic molecule that binds to the peptidyl transferase center of bacterial ribosomes, preventing protein synthesis; which identifies this compound as a potent antibiotic. While this molecule is structurally complex, a recent laboratory synthesis was completed using a simple cycloalkene as a starting material (shown below on the right). Ме но он Me- -H Me Me Me () Pleuromutilin contains many different functional groups. From the list below, identify all the functional groups present in the structure of pleuromutilin and label each functional group on the structure with the corresponding letter (see example). (a) alkyl group, (b) alkene, (c) alkyne, (d) 1° alkyl halide, (e) 2° alkyl halide, (1) 3° alkyl halide, (g) 1° alcohol, (h) 2° alcohol, (1) 3° alcohol, (j) ether, (K) epoxide, (1) aldehyde, (m) ketone, (n) carboxylic acid, (o) ester, (p) 1° amine, (a) 2° amine, (1) 3° amine, (s) imine, (1) nitrile (i) How many sp3…arrow_forwardWhat is the IUPAC name of the following molecule? Br Br (2R,4R)-dibromohexane (3R,5S)-dibromohexane (2R,4S)-dibromohexane O (R,S)-dibromohexane mirror imagearrow_forward
- 2. SN2 reactions to fight cancer Cyclophosphamide is a chemotherapy medication used to treat various types of cancers (i.e. lymphoma, leukemia, and breast cancer). It belongs to a group of cytotoxic alkylating agents known as Nitrogen mustards. The activity of these drugs is attributed to the chloroethylamine groups. CI R. 'N I R NH Cl + Cl R'-NH₂ Cyclophosphamide In this exercise, you will explore the mechanism responsible for the cytotoxic activity of Nitrogen mustards and how it is possible to tune their activity (therefore their toxicity) by modifying their chemical structure. R. 2.1 Identify and label all nucleophiles/electrophiles in the following reaction. `N I R chloroethylamine 2.2 Based on your above analysis, draw two possible SN2 reaction mechanisms Intermolecular reaction (reaction between reaction sites on different molecules) Intramolecular reaction (reaction between reaction sites within the same molecule) 2.3 Which of the above reaction will be faster? Why? 2.4 Copy…arrow_forwardChemistry 1) Using the (E)-(Z) designation [and in parts (e) and (f) the (R)-(S) designation as well] give IUPAC names for each of the following: (b) (c) HC (d) (e) ہو 3 CH, CH, Họ H H CH₂arrow_forward4 – Use bond-dissociation enthalpies (pg 167 of text) to calculate the DHo for each of the following reactions. CH3CH2CH3 + H2 g CH3CH3 + CH4 CH3CH2Cl + HI g CH3CH2I + HClarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





