
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 4, Problem 4.17P
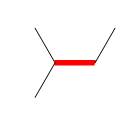
a. Draw the three staggered and three eclipsed conformations that
result from rotation around the bond labeled in red using Newman projections.
b. Label the most stable and least stable conformation.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ph
heat
heat
(12) Which one of the following statements about fluo-
rometry is FALSE?
a) Fluorescence is better detected at 90 from the exci-
tation direction.
b) Fluorescence is typically shifted to longer wave-
length from the excitation wavelength.
c) For most fluorescent compounds, radiation is pro-
duced by a
transition
Don't used Ai solution
Chapter 4 Solutions
Organic Chemistry
Ch. 4 - Prob. 4.1PCh. 4 - Problem 4.2 Which of the following is not another...Ch. 4 - Problem 4.3 Draw the five constitutional isomers...Ch. 4 - Prob. 4.4PCh. 4 - Prob. 4.5PCh. 4 - Draw the five constitutional isomers that have...Ch. 4 - Problem 4.7 Give the IUPAC name for each...Ch. 4 - Give the IUPAC name for each compound. a....Ch. 4 - Problem 4.9 Give the structure corresponding to...Ch. 4 - Prob. 4.10P
Ch. 4 - Give the IUPAC name for each compound.Ch. 4 - Give the structure corresponding to each IUPAC...Ch. 4 - Arrange the following compounds in order of...Ch. 4 - Problem 4.14 Draw the staggered and eclipsed...Ch. 4 - Prob. 4.15PCh. 4 - Prob. 4.16PCh. 4 - Problem 4.17 a. Draw the three staggered and...Ch. 4 - Problem 4.18 Rank the following conformations in...Ch. 4 - Problem 4.19 Consider rotation around the...Ch. 4 - Calculate the destabilization present in each...Ch. 4 - Problem 4.21 Classify the ring carbons as up or...Ch. 4 - Problem 4.22 Using the cyclohexane with the C’s...Ch. 4 - Draw a second chair conformation for each...Ch. 4 - Problem 4.24 Draw both conformations for and...Ch. 4 - Problem 4.25 Draw the structure for each compound...Ch. 4 - For cis-1, 3-diethylcyclobutane, draw a a...Ch. 4 - Prob. 4.27PCh. 4 - Problem 4.28 Consider .
Draw structures f or the...Ch. 4 - Problem 4.29 Draw a chair conformation of...Ch. 4 - Prob. 4.30PCh. 4 - Draw the products of each combustion reaction.Ch. 4 - Explain why beeswax is insoluble in H2O, slightly...Ch. 4 - Prob. 4.33PCh. 4 - Name each alkane using the ball-and-stick model,...Ch. 4 - Consider the substituted cyclohexane shown in the...Ch. 4 - Prob. 4.36PCh. 4 - Prob. 4.37PCh. 4 - 4.38 Give the IUPAC name for each compound.
a. c....Ch. 4 - 4.39 Give the structure and IUPAC name for each of...Ch. 4 -
4.40 Draw the structure corresponding to each...Ch. 4 - Prob. 4.41PCh. 4 - 4.42 Give the IUPAC name for each compound.
a....Ch. 4 - Prob. 4.43PCh. 4 - Prob. 4.44PCh. 4 - 4.45 Which conformation in each pair is higher in...Ch. 4 - 4.46 Considering rotation around the bond...Ch. 4 - Prob. 4.47PCh. 4 - 4.48 (a) Using Newman projections, draw all...Ch. 4 - 4.49 Label the sites of torsional and steric...Ch. 4 - 4.50 Calculate the barrier to rotation for each...Ch. 4 - 4.51 The eclipsed conformation of is less...Ch. 4 - (a) Draw the anti and gauche conformations for...Ch. 4 - For each compound drawn below: a.Label each OH,Br...Ch. 4 - Draw the two possible chair conformations for...Ch. 4 - For each compound drawn below: a. Draw...Ch. 4 - 4.56 Convert each of the following structures into...Ch. 4 - Prob. 4.57PCh. 4 - Prob. 4.58PCh. 4 - 4.59 Classify each pair of compounds as...Ch. 4 - Classify each pair of compounds as constitutional...Ch. 4 - Prob. 4.61PCh. 4 - 4.62 Draw the three constitutional isomers having...Ch. 4 - Prob. 4.63PCh. 4 - 4.64 Draw the products of combustion of each...Ch. 4 - 4.65 Hydrocarbons like benzene are metabolized in...Ch. 4 - Prob. 4.66PCh. 4 - Prob. 4.67PCh. 4 - Cyclopropane and cyclobutane have similar strain...Ch. 4 - Prob. 4.69PCh. 4 - Haloethanes (CH3CH2X,X=Cl,Br,I) have similar...Ch. 4 - Prob. 4.71PCh. 4 - Prob. 4.72PCh. 4 - Consider the tricyclic structure B (a) Label each...Ch. 4 - Read Appendix B on naming branched alkyl...Ch. 4 - Read Appendix B on naming bicyclic compounds. Then...
Additional Science Textbook Solutions
Find more solutions based on key concepts
2. Which of the following is the best example of the use of a referent? _
a. A red bicycle
b. Big as a dump tru...
Physical Science
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Describe the role and impact of microbes on the earth.
Microbiology Fundamentals: A Clinical Approach
On what molecule does the anticodon appear? Explain the role of this molecule in protein synthesis.
Human Physiology: An Integrated Approach (8th Edition)
Why is it necessary to be in a pressurized cabin when flying at 30,000 feet?
Anatomy & Physiology (6th Edition)
1. Genetics affects many aspects of our lives. Identify three ways genetics affects your life or the life of a ...
Genetic Analysis: An Integrated Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License