
(a)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(a)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
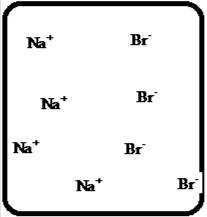
Figure 1
Let us consider the above molecular-level pictures of
number of
(b)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(b)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,.
Explanation of Solution
To draw the molecular-level pictures of
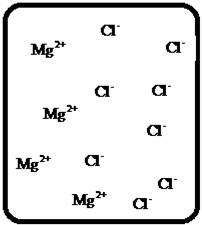
Figure 2
Let us consider the above molecular-level pictures of
number of
(c)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(c)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
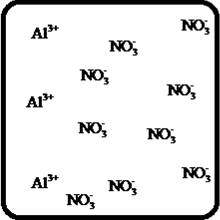
Figure 3
Let us consider the above molecular-level pictures of
number of
should show equal
number of
(d)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(d)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
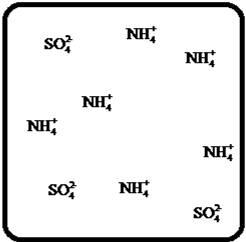
Figure 4
Let us consider the above molecular-level pictures of
number of
(e)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(e)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
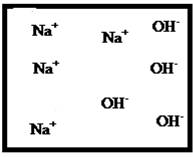
Figure 5
Let us consider the above molecular-level pictures of
Number of
(f)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(f)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
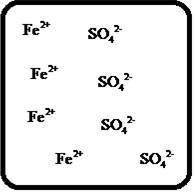
Figure 6
Let us consider the above molecular-level pictures of
number of
(g)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(g)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
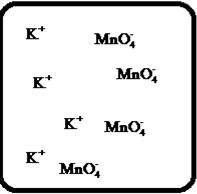
Figure 7
Let us consider the above molecular-level pictures of
number of
(h)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(h)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
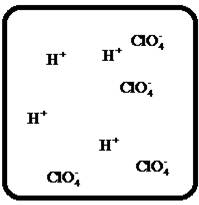
Figure 8
Let us consider the above molecular-level pictures of
number of
(i)
Interpretation:
Drawing the molecular-level pictures of strong electrolytes when its breaks up into component ions upon dissolving in water.
Concept Introduction:
Strong electrolyte totally dissociates in a solution. These ions are good conductors of emotional current in the solution.
(i)
Answer to Problem 27E
The dissociation of the given strong electrolyte is,
Explanation of Solution
To draw the molecular-level pictures of
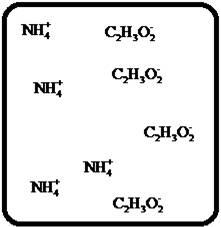
Figure 9
Let us consider the above molecular-level pictures of
Number of
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry
- What volume of 0.250 M HCI is required to neutralize each of the following solutions? a. 25.0 mL of 0.103 M sodium hydroxide, NaOH b. 50.0 mL of 0.00501 M calcium hydroxide, Ca(OH)2 c. 20.0 mL of 0.226 M ammonia, NH3 d. 15.0 mL of 0.0991 M potassium hydroxide, KOHarrow_forwardThe (aq) designation listed after a solute indicates the process of hydration. Using KBr(aq) and C2H5OH(aq) as your examples, explain the process of hydration for soluble ionic compounds and for soluble covalent compounds.arrow_forwardssume a highly magnified view of a solution of HCI that allows you to “see” the HCl. Draw this magnified view. If you dropped in a piece of magnesium, the magnesium would disappear, and hydrogen gas would he released. Represent this change using symbols for the elements, and write the balanced equation.arrow_forward
- Match each name below with the following microscopic pictures of that compound in aqueous solution. a. barium nitrate b. sodium chloride c. potassium carbonate d. magnesium sulfate Which picture best represents HNO3(aq)? Why arent any of the pictures a good representation of HC2H3O2(aq)?arrow_forwardVitamin C has the formula C6H8O6. Besides being an acid, it is a reducing agent. One method for determining the amount of vitamin C in a sample is to titrate it with a solution of bromine, Br2, an oxidizing agent. C6H8O6(aq) + Br2(aq) 2 HBr(aq) + C6H6O6(aq) A 1.00-g "chewable" vitamin C tablet requires 27.85 ml of 0.102 M Br2 for titration to the equivalence point. What is the mass of vitamin C in the tablet?arrow_forwardefine the term strong electrolyte. What types of substances tend to be strong electrolytes? What does a solution of a strong electrolyte contain? Give a way to determine if a substance is a strong electrolyte.arrow_forward
- Reactions represented by the following equations take place in water solutions. Write each molecular equation in total ionic form, then identify spectator ions and write the equations in net ionic form. Solids that do not dissolve are designated by s, gases that do not dissolve are designated by g, and substances that dissolve but do not dissociate appear in blue. a. H2O(l)+Na2SO3(aq)+SO2(aq)2NaHSO3(aq) b. 3Cu(s)+8HNO3(aq)3Cu(NO3)2(aq)+2NO(g)+4H2O(l) c. 2HCl(aq)+CaO(s)CaCl2(aq)+H2O(l) d. CaCO3(s)+2HCl(aq)CaCl2(aq)+CO2(aq)+H2O(l) e. MnO2(s)+4HCl(aq)MnCl2(aq)+Cl2(aq)+2H2O(l) f. 2AgNO3(aq)+Cu(s)Cu(NO3)2(aq)+2Ag(s)arrow_forwardA 10.00-mL sample of a 24.00% solution of ammonium bromide (NH4Br) requires 23.41 mL of 1.200 molar silver nitrate (AgNO3) to react with all of the bromide ion present. (a) Calculate the molarity of the ammonium bromide solution. (b) Use the molarity of the solution to find the mass of ammonium bromide in 1.000 L of this solution. (c) From the percentage concentration and the answer to part b, find the mass of 1.000 L ammonium bromide solution. (d) Combine the answer to part c with the volume of 1.000 L to express the density of the ammonium bromide solution (in g/mL).arrow_forwardDescribe some physical and chemical properties of acids and bases. What is meant by a strong acid or base? Are strong acids and bases also strong electrolytes? Give several examples of strong acids and strong bases.arrow_forward
- Lead poisoning has been a hazard for centuries. Some scholars believe that the decline of the Roman Empire can be traced, in part, to high levels of lead in water from containers and pipes, and from wine that was stored in leadglazed containers. If we presume that the typical Roman water supply was saturated with lead carbonate, PbCO3 (Ksp = 7.4 1014), how much lead will a Roman ingest in a year if he or she drinks 1 L/day from the container?arrow_forwardA mountain lake that is 4.0 km × 6.0 km with an average depth of 75 m has an H+(aq) concentration of 1.3 × 10−6 M. Calculate the mass of calcium carbonate that would have to be added to the lake to change the H+(aq) concentration to 6.3 × 10−8 M. Assume that all the carbonate is converted to carbon dioxide, which bubbles out of the solution.arrow_forwardWhen 10. L of water is added to 3.0 L of 6.0 M H2SO4, what is the molarity of the resulting solution? Assume the volumes are additive.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





