
Pushing Electrons
4th Edition
ISBN: 9781133951889
Author: Weeks, Daniel P.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 3, Problem 7EQ
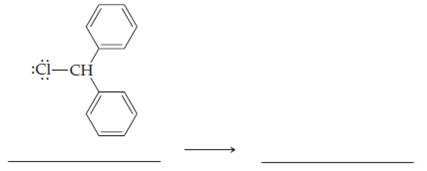
Here are some exercises in sigma bond breaking. Supply the arrows and the products. Remember that charge must be conserved. That is, if a neutral molecule is dissociated, the algebraic sum of the charges on the products must equal zero. If a positively charged ion is dissociated, the algebraic sum of the charges on the products must equal +1.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Frenkel and Schottky are intrinsic or extrinsic defects, point or linear defects.
Select the correct option:a) Frenkel and Schottky defects are linear crystal defects.b) Schottky defects involve atomic motions in a crystal lattice.c) Frenkel defects are vacancies in a crystal lattice.d) None of the above is correct.
The most common frequency in organic chemistry is the
Select one:
Oa. carbon-oxygen single bond
Ob. None of the above
Oc.
carbon-carbon double bond
Od. carbon-carbon single bond
Chapter 3 Solutions
Pushing Electrons
Ch. 3 - Prob. 1EQCh. 3 - Prob. 2EQCh. 3 - Prob. 3EQCh. 3 - Prob. 4EQCh. 3 - Prob. 5EQCh. 3 - Prob. 6EQCh. 3 - Here are some exercises in sigma bond breaking....Ch. 3 - Prob. 8EQCh. 3 - Prob. 9EQCh. 3 - Prob. 10EQ
Ch. 3 - Prob. 11EQCh. 3 - Prob. 12EQCh. 3 - Prob. 13EQCh. 3 - Prob. 14EQCh. 3 - Prob. 15EQCh. 3 - Prob. 16EQCh. 3 - Prob. 17EQCh. 3 - Prob. 18EQCh. 3 - Prob. 19EQCh. 3 - Prob. 20EQCh. 3 - Prob. 21EQCh. 3 - Prob. 22EQCh. 3 - Prob. 23EQCh. 3 - Prob. 24EQCh. 3 - Prob. 25EQCh. 3 - Prob. 26EQCh. 3 - Prob. 27EQCh. 3 - Prob. 28EQCh. 3 - Prob. 29EQCh. 3 - Prob. 30EQCh. 3 - The reaction just described is reversible....Ch. 3 - Prob. 32EQCh. 3 - Prob. 59EQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nonearrow_forwardUnshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. CH. H₂ fo H2 H The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is HC HC HC CH The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c isarrow_forwardDraw curved arrows for the following reaction step. Arrow-pushing Instructions CH3 CH3 H H-O-H +/ H3C-C+ H3C-C-0: CH3 CH3 Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY