
Concept explainers
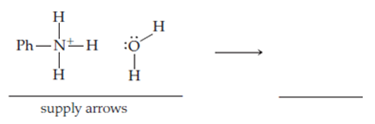
The reaction just described is reversible. Deprotonation of the conjugate acid of an organic base by water provides another example of simultaneous making and breaking of sigma bonds. Thus, in the deprotonation of anilinium ion by water, the base is water, which has unshared electrons on the ________ atom. The acid is ________ ion. A pair of ________ electrons on the oxygen atom of water is pushed toward the ________ atom. Simultaneously, the pair of ________ electrons between the hydrogen and ________ atom of the anilinium ion is pushed toward the ________ atom. Thus, the oxygen- ________ sigma bond is made and a hydrogen- ________ sigma bond is broken. The nitrogen atom, which possessed a positive charge, is now ________, and the oxygen atom, which was neutral, now possesses a formal ________ charge.
Trending nowThis is a popular solution!

Chapter 3 Solutions
Pushing Electrons
- Perchloric acid is ______(more/less)______ stable after donating a hydrogen ion due to____(resonance stability / polarizability or smaller atomic size) ______.arrow_forwardAn aqueous solution of CH3NH3NO3 will be Group of answer choices A. basic, because of the hydrolysis of NO3– ions. B. acidic, because of the hydrolysis of NO3– ions. C. acidic, because of the hydrolysis of CH3NH3+ ions. D. basic, because of the ionization of CH3NH2. E. basic, because of the hydrolysis of CH3NH3+ ions.arrow_forward4. Calculate the pH of a 0.100 M solution of alanine prepared from the form shown below. pKa (-COOH) = 2.344 and pKa (-NH;") = 9.868. H *H;N- C C O- CH3 pH = 6.106 5. Referring to Question 4, calculate the concentration of the fully protonated form of alanine in the solution described in Question 4. [H2A*] = 1.72*10-$ Marrow_forward
- Calculate the volume of HCl, of concentration 0,5 mol.dm–3, thatis required to completely neutralize 25 cm3 of a NaOH solutionhaving a pH of 13 at 25 degree Celsius . _______________________________________arrow_forwardQuestion 9 of 12 Bra A H20 1. LIAIH. B 2. H3O* PBra DMF conc. HBr но D но H30* Donearrow_forwardFor Exercise 40-41, use the formula pH = -log[H*] to compute the pH of a liquid as a function of its concentration of hydronium ions, [H'] in mol/L. If the pH is less than 7, then the substance is acidic. If the pH is greater than 7, then the substance is alkaline (or basic). a. Find the pH. Round to 1 decimal place. b. Determine whether the substance is acidic or alkaline. 40. Baking soda: [H*] = 5.0 x 10° mol/L 41. Tomatoes: [H*] = 3.16 x 10-5 mol/Larrow_forward
- Complete the following table "predicted pH." Please show all work on a separate sheet of paper and attach. Note that the pK, of H₂PO4 is 7.2. Actual pH Predicted pH Original After 45 µL of 5 M NaOH solution 4.30 6.05 After 205 μL more of 5M NaOH 6.90 After 205 μL more of 5M NaOH 11.01 After 45 μL more of 5 M NaOH 11.29arrow_forwardThe container contains formic acid HCOOH as a 0.10 mol/dm3 solution, and the oxonium ion concentration [H3O+] of the solution is 4.08 mmol/dm3. What is the Ka value of the acid constant? a. 1,7 * 10-4 mol / l b. 0,65 * 10-2 mol / l c. 1,7 mol / larrow_forwardA carboxylic acid has a pKa of 6.00. Which of the following statements is true? O A. There is an equimolar ratio of the acid and its conjugate base when the pH is 6.00. B. Half of the acid will be deprotonated when the pH = 3. O C. Half of the acid will be protonated when the pH = 12. D. The acid will be completely neutralized when the pH = 7.00. %3!arrow_forward
- H3C—N̈H2 reacts with BF3 in a combination reaction, forming H3C—NH2—BF3 with a covalent bond between N and B. What is the role of H3C—N̈H2 in this reaction? a. Lewis acid b. Brønsted-Lowry base c. Brønsted-Lowry acid d. Lewis basearrow_forwardb My Questions | bartleby rellum.ecollege.com/course.html?courseld%316519516&OpenVellumHMAC=Da14e8ada06537be6b0 Best Free PowerP... L Google Drive Academic Search I Downloads ouTube Maps Submit Request Answer Part G Write the formula of the conjugate acid for base Br. Express your answer as a chemical formula or an ion. NA chemical reaction does not occur for this question. Submit Request Answer Part H Write the name of the conjugate acid found in part G. Spell out the full name of the compound. Submit Request Answerarrow_forwardrch ||| O CHEMICAL REACTIONS Predicting the products of a neutralization reaction Predict the products of the reaction below. That is, complete the right-hand side of the chemical equation. Be sure your equation is balanced. HNO3 + NaOH → 0 Explanation Ease RK- burial WE burial Check papers 46 H. Kdy go ahead X 09 1/5 Christopher Mitchell Jessica V 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 000 14 GEarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





