
Concept explainers
(a)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(a)
Answer to Problem 59A
The element with the given chemical symbol,
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
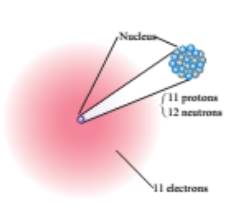
From the given figure, the first element contains
The atomic number of the element is
Hence, the first element is
(b)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(b)
Answer to Problem 59A
The elements with the chemical symbol and atomic number and mass number -
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
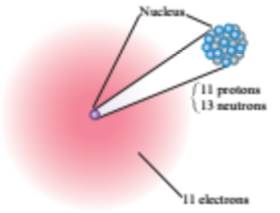
From the given figures
The second element contains
The atomic number of the element is
Hence, the second element is
(c)
Interpretation:
Using the
Concept Introduction:
Every matter is made of chemicals. Chemistry is very important for the study of everything. Chemistry is all about how something interacts to affect the structure, composition, as well as properties of substances.
(c)
Answer to Problem 59A
The element with the chemical symbol and atomic number and mass number is:
Explanation of Solution
Protons are defined as positively charged
Electrons are negatively charged subatomic particles found orbiting the nucleus of an atom.
For neutral atom, the number of Electron = number of protons.
Neutrons are subatomic particles found in the nucleus of an atom which is neutral.
An atom has a nucleus with a positive charge due to its protons and has electrons in the space surrounding the nucleus at a relatively large distance from it.
For
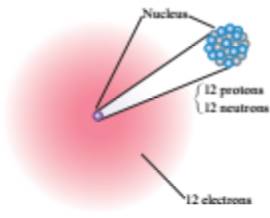
The element contains
The atomic number of the element is
And the mass number is
Hence the third element is
Chapter 3 Solutions
World of Chemistry, 3rd edition
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





