
Study Guide for Campbell Biology
11th Edition
ISBN: 9780134443775
Author: Lisa A. Urry, Michael L. Cain, Steven A. Wasserman, Peter V. Minorsky, Jane B. Reece, Martha R. Taylor, Michael A. Pollock
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 4IQ
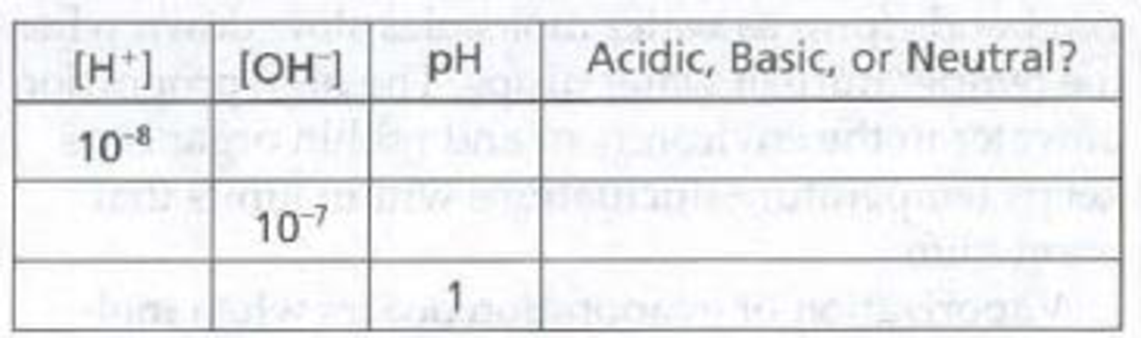
Complete the following table to review your understanding of pH.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Label the image of the PH scale using the terms
A small amount of NaOH is added to a weak acid buffer system. Describe what happens in terms of possible reactions and changes in pH.
For the questions below be sure to show your work as well as the answer to the question.
In this week's lab you made 100ml of 10x PBS by dissolving 8 g NaCl, 1.2g Na₂HPO4 and 0.2 g
KH₂PO4 in water. You then adjusted the pH to 7.0.
A) How many mg of KH₂PO4 did you weigh out?
B) How many liters of 10x PBS did you make?
C) What is the final concentration of NaCl in your solution? (The molecular weight of NaCl is
58.44 g/mol)
D) What is the final concentration of Na₂HPO4 in your solution? (The molecular weight of
Na₂HPO4 is 141.96 g/mol)
E) What is the final concentration of KH₂PO4 in your solution? (The molecular weight of KH₂PO4
is 136.1 g/mol)
F) What is the hydrogen ion concentration of your solution (pH=7.0)?
G) If the initial pH of your solution was 6.29 would you need to add an acid or a base to adjust
the pH of a solution to pH 7.0?
10x PBS is a concentrated stock solution that is used to make more dilute solutions of PBS.
Starting with your 10xPBS stock solution, how would you…
Chapter 3 Solutions
Study Guide for Campbell Biology
Ch. 3 - Draw the four water molecules that can...Ch. 3 - The following concept map is one way to show how...Ch. 3 - Prob. 3IQCh. 3 - Complete the following table to review your...Ch. 3 - Prob. 5IQCh. 3 - a. Add to the formula in Interactive Question 3.5...Ch. 3 - Fill in the following table, which summarizes the...Ch. 3 - To become proficient in the use of the concepts...Ch. 3 - Each water molecule is capable of forming a. three...Ch. 3 - The properties of water make it an ideal and...
Ch. 3 - What accounts for the movement of water up the...Ch. 3 - Climates tend to be moderate near large bodies of...Ch. 3 - You have three flasks containing 100 mL of...Ch. 3 - A burn from steam at 100C is more severe than a...Ch. 3 - Evaporative cooling is a result of a. the release...Ch. 3 - Prob. 8TYKCh. 3 - Prob. 9TYKCh. 3 - Prob. 10TYKCh. 3 - Prob. 11TYKCh. 3 - Prob. 12TYKCh. 3 - Prob. 13TYKCh. 3 - Consider two solutions labeled A and B. Solution A...Ch. 3 - What does a buffer do? a. moderates pH changes b....Ch. 3 - Prob. 16TYKCh. 3 - Prob. 17TYKCh. 3 - Prob. 18TYK
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Cite possible research study wherein detecting the pH of a substance is an essential procedure in the study.arrow_forwardBuffers also exist in biological systems. Discuss the composition and function of one example of a biological buffer.arrow_forwardName three vital properties of water in living cells.arrow_forward
- Define a buffer and explain how a buffer works. [Note: be sure to address the role of conjugate salts.] Analyze and explain the buffer system in buffered aspirin (carboxylic acid).arrow_forwardUsing a ph meter you find the ph of an unknown solution to be 8.0. How would you describe this solution ?arrow_forwardMake a list of the five most critical qualities of an effective extraction buffer. Please provide a brief description of each.arrow_forward
- In biological systems, buffers are important because they facilitate changes in pH to enhance biological activities of proteins and nucleic acids. True Falsearrow_forwardUsing an example compound, describe the role of buffers in regulating pH.arrow_forwardDefine the term buffer. Explain the difference between carbonic acid (H2CO3) and hydrochloric acid (HCl). Can they both be used as buffers? Why or why not.arrow_forward
- The negative logarithm of the hydrogen ion concentration of an aqueous solution defines the of that solution. (answer case-sensitive) none of the above basic alkaline O pHarrow_forwardA depressed person attempts to commit suicide by taking a whole bottle of sleeping pills. The pills cause a drastic decrease in the pH. Explain in depth the correlation between the person’s pHarrow_forwardWhat lab values are the hardest to understand within the concept of fluid and electrolytes?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning

Human Biology (MindTap Course List)
Biology
ISBN:9781305112100
Author:Cecie Starr, Beverly McMillan
Publisher:Cengage Learning
GCSE Chemistry - Acids and Bases #34; Author: Cognito;https://www.youtube.com/watch?v=vt8fB3MFzLk;License: Standard youtube license