
General, Organic, and Biochemistry
9th Edition
ISBN: 9780078021541
Author: Katherine J Denniston, Joseph J Topping, Dr Danae Quirk Dorr
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 3.91QP
(a)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
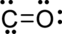
(b)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
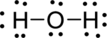
(c)
Interpretation Introduction
Interpretation:
The error in the given Lewis structure has to be found and the correct structures has to be written.
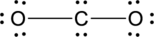
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3. Titanium(III) chloride can be used to catalyze the polymerization of ethylene. It is prepared by hydrogen reduction of
Titanium(IV) chloride. Reaction of hydrogen gas with titanium(IV) chloride gas produces solid titanium(III) chloride and
hydrogen chloride gas.
(a) Write a BALANCED chemical reaction for the preparation of titanium(III) chloride
(b) A 250 L reaction vessel at 325°C is filled with hydrogen gas to a pressure of 1.3 atm. Titanium(IV) chloride is then added
to bring the total pressure to 3.00 atm. How many grams of titanium(III) chloride will be produced after completion of the
reaction?
(c) What will be the pressure of the resulting hydrogen chloride gas that is also produced?
1. Sodium azide (NaN3) is the primary chemical substance used in automobile air bags. Upon impact, the decomposition of
sodium azide is initiated to produce sodium metal and nitrogen gas which then inflates the bag. How many liters of
nitrogen gas are produced at 1.15 atm and 30.0°C when 145.0 grams of sodium azide decomposes?
2. Calcium carbonate (such as that in limestone) reacts with aqueous hydrochloric acid to produce carbon dioxide, aqueous
calcium chloride and water. How many liters of carbon dioxide are produced at 20°C and 745 torr when 3.583 grams of
calcium carbonate is dissolved in solution containing 1.550 grams of hydrochloric acid?
Show all work (where appropriate) for full credit.
1. Describe (steps, equipment and quantities) how to accurately prepare 250.0 mL of a 0.0075
M solution of NaCl (aq) from a 500 mL, 0.0500 M stock solution.
2. Describe (steps, equipment and quantities) how to accurately prepare 250.0 mL of a 0.0075
M solution of NaCl (aq) from 100 g of solid NaCl.
Chapter 3 Solutions
General, Organic, and Biochemistry
Ch. 3.1 - Draw the Lewis symbol for oxygen, and indicate the...Ch. 3.1 - Prob. 3.2PPCh. 3.1 - Prob. 3.3PPCh. 3.2 - Prob. 3.4PPCh. 3.2 - Prob. 3.5PPCh. 3.2 - Prob. 3.1QCh. 3.2 - Prob. 3.2QCh. 3.2 - Prob. 3.6PPCh. 3.2 - Prob. 3.7PPCh. 3.2 - Prob. 3.8PP
Ch. 3.2 - Prob. 3.9PPCh. 3.4 - Prob. 3.10PPCh. 3.4 - Prob. 3.11PPCh. 3.4 - Prob. 3.12PPCh. 3.4 - Prob. 3.13PPCh. 3.4 - Prob. 3.3QCh. 3.4 - Prob. 3.4QCh. 3.4 - Prob. 3.14PPCh. 3.4 - Prob. 3.5QCh. 3.4 - Prob. 3.6QCh. 3.4 - Prob. 3.16PPCh. 3.4 - Prob. 3.7QCh. 3.4 - Prob. 3.8QCh. 3.4 - Prob. 3.9QCh. 3 - Prob. 3.13QPCh. 3 - Draw the appropriate Lewis symbol for each of the...Ch. 3 - Draw the appropriate Lewis symbol for each of the...Ch. 3 - Prob. 3.16QPCh. 3 - Describe the differences between covalent bonding...Ch. 3 - Describe the difference between nonpolar covalent...Ch. 3 - What is the periodic trend of electronegativity?
Ch. 3 - What role does electronegativity play in...Ch. 3 - Use electronegativity values to classify the bonds...Ch. 3 - Use electronegativity values to classify the bonds...Ch. 3 - When there is a reaction between each of these...Ch. 3 - Prob. 3.24QPCh. 3 - Explain, using Lewis symbols and the octet rule,...Ch. 3 - Explain, using Lewis symbols and the octet rule,...Ch. 3 - Prob. 3.27QPCh. 3 - Prob. 3.28QPCh. 3 - Name each of the following ions:
Na+
Cu+
Mg2+
Ch. 3 - Name each of the following ions:
Cu2+
Fe2+
Fe3+
Ch. 3 - Name each of the following ions:
HCO3–
H3O+
CO32−
Ch. 3 - Prob. 3.32QPCh. 3 - Prob. 3.33QPCh. 3 - Write the formula for each of the following...Ch. 3 - Prob. 3.35QPCh. 3 - Prob. 3.36QPCh. 3 - Prob. 3.37QPCh. 3 - Predict the formula of a compound formed...Ch. 3 - Prob. 3.39QPCh. 3 - Prob. 3.40QPCh. 3 - Prob. 3.41QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Prob. 3.43QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Prob. 3.45QPCh. 3 - Write the correct formula for each of the...Ch. 3 - Write a suitable formula for:
sodium...Ch. 3 - Write a suitable formula for:
aluminum...Ch. 3 - Prob. 3.49QPCh. 3 - Prob. 3.50QPCh. 3 - Prob. 3.51QPCh. 3 - Prob. 3.52QPCh. 3 - Write a suitable formula for:
silicon...Ch. 3 - Prob. 3.54QPCh. 3 - Contrast ionic and covalent compounds with respect...Ch. 3 - Prob. 3.56QPCh. 3 - Prob. 3.57QPCh. 3 - Prob. 3.58QPCh. 3 - Prob. 3.59QPCh. 3 - Prob. 3.60QPCh. 3 - Prob. 3.61QPCh. 3 - Prob. 3.62QPCh. 3 - Prob. 3.63QPCh. 3 - Prob. 3.64QPCh. 3 - Prob. 3.65QPCh. 3 - Prob. 3.66QPCh. 3 - How is the positive charge of a polyatomic cation...Ch. 3 - Prob. 3.68QPCh. 3 - Prob. 3.69QPCh. 3 - Prob. 3.70QPCh. 3 - Prob. 3.71QPCh. 3 - Prob. 3.72QPCh. 3 - Prob. 3.73QPCh. 3 - Prob. 3.74QPCh. 3 - Prob. 3.75QPCh. 3 - Prob. 3.76QPCh. 3 - Prob. 3.77QPCh. 3 - Prob. 3.78QPCh. 3 - Prob. 3.79QPCh. 3 - Prob. 3.80QPCh. 3 - Prob. 3.81QPCh. 3 - Prob. 3.82QPCh. 3 - Prob. 3.83QPCh. 3 - Prob. 3.84QPCh. 3 - Prob. 3.85QPCh. 3 - Prob. 3.86QPCh. 3 - Prob. 3.87QPCh. 3 - Prob. 3.88QPCh. 3 - Prob. 3.89QPCh. 3 - Prob. 3.90QPCh. 3 - Prob. 3.91QPCh. 3 - Prob. 3.93QPCh. 3 - Prob. 3.94QPCh. 3 - Prob. 3.95QPCh. 3 - Prob. 3.96QPCh. 3 - Prob. 3.97QPCh. 3 - Prob. 3.98QPCh. 3 - Prob. 3.99QPCh. 3 - Prob. 3.100QPCh. 3 - Prob. 3.101QPCh. 3 - Prob. 3.102QPCh. 3 - Prob. 3.103QPCh. 3 - Prob. 3.104QPCh. 3 - Prob. 3.107QPCh. 3 - Prob. 3.108QPCh. 3 - Prob. 3.109QPCh. 3 - Prob. 3.110QPCh. 3 - Prob. 3.112QPCh. 3 - Predict differences in our global environment that...Ch. 3 - Prob. 2CPCh. 3 - Prob. 3CPCh. 3 - Prob. 4CPCh. 3 - Prob. 5CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5. An unlabeled gas cylinder was recently found in the laboratory. A sample of the gas was removed and analyzed. A 500.0 mL sample of the gas at 15°C and a pressure of 736 mmHg was found to weigh 2.688 g. Determine the molar mass of the gas. What element is the gas?arrow_forward4. Nitrogen gas is commonly sold in 49.0 L steal cylinders at a pressure of 150 atm. (a) How many moles of nitrogen are in the container if the temperature of the cylinder is 21°C. (b) How many moles of nitrogen will there be if the container above is heated to 100°C? (careful here) (c) What is the mass of nitrogen gas in the cylinder in part (a)? (d) What volume would the nitrogen occupy at 21°C, if the pressure was reduced to 1.02 atm? (e) What would be the pressure of the nitrogen gas in the cylinder when the temperature is raised to 39°C?arrow_forward6. A 0.4550 g sample of an unknown organic compound with the empirical formula CH2O was placed into a 150.0 ml vessel and was vaporized into a gas. At 175.0°C, the pressure of the vaporized compound was measured at 941.1 torr. (a) Determine the molar mass of the compound (b) Determine the molecular formula of the compound.arrow_forward
- Don't used Ai solutionarrow_forward3. A particular reaction calls for 2.40 g of chloride ion. The only source of chloride ion available is a 0.00300 M stock solution of strontium chloride. How much (in L) of this solution is needed for this reaction?arrow_forwardAbsorption Spectrum of NaphthaleneTitle: Understanding the Absorption Spectrum of NaphthaleneGraph: Show a graph with labeled peaks indicating the absorption spectrum of naphthalene in a suitable solventarrow_forward
- Show work...don't give Ai generated solutionarrow_forwardShow work..don't give Ai generated and copy the answer anywhere.arrow_forwardthis is an inorganic chemistry question please answer accordindly!! its just one question with parts till (n) JUST ONE QUESTION with its parts spread out in the form of different images attached 2 IMAGES ATTACHED PLEASE SEE BOTH, please answer EACH part till the end and dont just provide wordy explanations wherever asked for structures, graphs or diagrams, please DRAW DRAW them on a paper and post clearly!! answer the full question with all details as needed EACH PART CLEARLY please or let another expert handle it thanks!! im reposting this please solve all parts and drawit not just word explanations!!arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY