
(a)
Interpretation:
The total number of subatomic particles present in
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
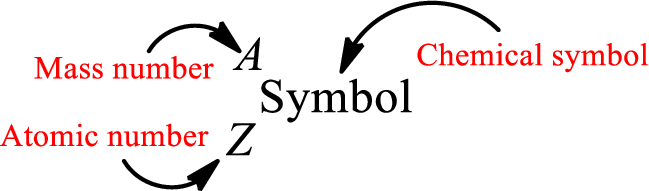
An element is a pure substance that cannot be broken by ordinary
(a)
Explanation of Solution
Given chemical symbol is
The total subatomic particles present in
(b)
Interpretation:
The total number of subatomic particles present nucleus of
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
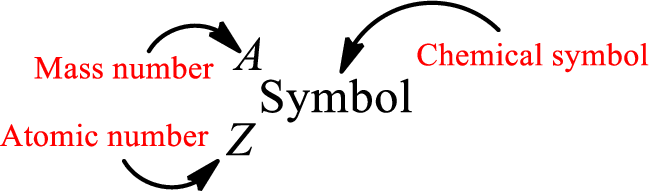
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(b)
Explanation of Solution
Given chemical symbol is
The total subatomic particles present in nucleus of
(c)
Interpretation:
The total number nucleons present in
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
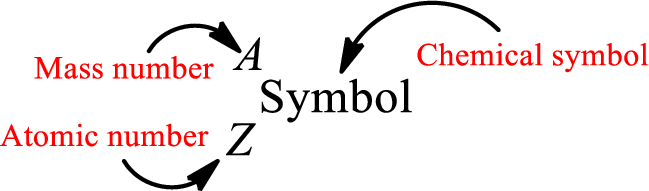
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(c)
Explanation of Solution
Given chemical symbol is
The total number of nucleons present in
(d)
Interpretation:
The total charge that is associated with nucleus of
Concept Introduction:
Atoms are made up of even smaller particles. These particles are very small and these are all the building blocks of atoms and they are known as subatomic particles. Protons, electrons, and neutrons are the subatomic particles that are found in atom. Electrons possess a negative electrical charge. Protons possess a positive electrical charge. Neutrons possess no charge and they are neutral.
Atomic number for each and every element is a unique one. This is the total number of protons that is present in an atom. As the atom is electrically neutral, it can also be said that the total number of electrons is the atomic number. Atomic number is represented by the symbol Z.
Mass number is the sum of the number of protons and neutrons inside the nucleus of an atom. This gives the number of subatomic particle present inside the nucleus. Mass number is represented by the symbol A.
From atomic number and mass number, the number of each sub atomic particle can be found.
Complete chemical symbol notation can be given as.
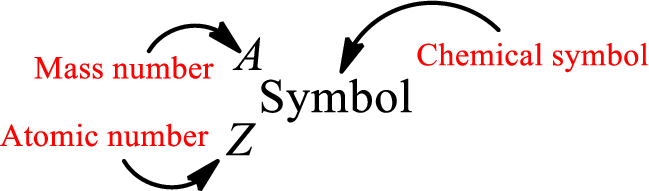
An element is a pure substance that cannot be broken by ordinary chemical reactions into simpler substances. All the atoms in an element will have the same atomic number. The electrons only take part in the chemical reaction while the nucleus does not. Hence, the atomic number (number or protons) does not change and it characterizes an atom.
(d)
Explanation of Solution
Given chemical symbol is
The total charge associated with the nucleus of
Want to see more full solutions like this?
Chapter 3 Solutions
Study Guide with Selected Solutions for Stoker's General, Organic, and Biological Chemistry, 7th
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning  Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





