
Concept explainers
Decide whether each of the following is water-soluble. If soluble, tell what ions are produced when the compound dissolves in water.
(a) NiCl2
(b) Cr(NO3)3
(c) Pb(NO3)2
(d) BaSO4
(a)
Interpretation:
Water solubility of the given compounds and ions produced by the compounds should be analyzed.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of (Na+), (K+), (NH4+), (NO3-), (ClO3-), (ClO4-)and(CH3CO2-) are soluble.
Almost all the salts of Cl-, Br-, I-(halides) are soluble. But some of the halides (Ag+, Hg22+, Pb2+) are insoluble.
Salts of F- are soluble. But some of the fluoride salt of Mg2+, Ca2+, Sr2+, Ba2+, Pb2+ are insoluble.
Salts of sulfate (SO42-) are soluble. But sulfates of Ca2+, Sr2+, Ba2+, Pb2+, Ag+, are insoluble.
Insoluble compounds in water:
Most of the salts of (CO32-), (PO43-), (C2O42-), (CrO42-) and(S2-) are insoluble but some of the salts of NH4+, alkali metal cations and BaS are soluble.
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides, Ba(OH)2and Sr(OH)2 are soluble in water.
Answer to Problem 24PS
Water soluble compound. Produced ions are Ni2+and Cl-.
Explanation of Solution
The given compound Nickel (II) chloride which is soluble in water.
NiCl2 is dissolves in water, Nickel(II) chloride is composed of Ni2+and Cl-, and it became ions in water.
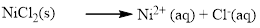
(b)
Interpretation:
Water solubility of the given compounds and ions produced by the compounds should be analyzed.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of (Na+), (K+), (NH4+), (NO3-), (ClO3-), (ClO4-)and(CH3CO2-) are soluble.
Almost all the salts of Cl-, Br-, I-(halides) are soluble. But some of the halides (Ag+, Hg22+, Pb2+) are insoluble.
Salts of F- are soluble. But some of the fluoride salt of Mg2+, Ca2+, Sr2+, Ba2+, Pb2+ are insoluble.
Salts of sulfate (SO42-) are soluble. But sulfates of Ca2+, Sr2+, Ba2+, Pb2+, Ag+, are insoluble.
Insoluble compounds in water:
Most of the salts of (CO32-), (PO43-), (C2O42-), (CrO42-) and(S2-) are insoluble but some of the salts of NH4+, alkali metal cations and BaS are soluble.
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides, Ba(OH)2and Sr(OH)2 are soluble in water.
Answer to Problem 24PS
Water soluble compound. Produced ions are Cr3+and NO3-.
Explanation of Solution
The given compound is Chromium (III) nitrate which is soluble in water.
Cr(NO3)3 is dissolves in water, Chromium(III) nitrate is composed of Cr3+and NO-3, and it became ions in water.
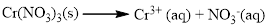
(c)
Interpretation:
Water solubility of the given compounds and ions produced by the compounds should be analyzed.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of (Na+), (K+), (NH4+), (NO3-), (ClO3-), (ClO4-)and(CH3CO2-) are soluble.
Almost all the salts of Cl-, Br-, I-(halides) are soluble. But some of the halides (Ag+, Hg22+, Pb2+) are insoluble.
Salts of F- are soluble. But some of the fluoride salt of Mg2+, Ca2+, Sr2+, Ba2+, Pb2+ are insoluble.
Salts of sulfate (SO42-) are soluble. But sulfates of Ca2+, Sr2+, Ba2+, Pb2+, Ag+, are insoluble.
Insoluble compounds in water:
Most of the salts of (CO32-), (PO43-), (C2O42-), (CrO42-) and(S2-) are insoluble but some of the salts of NH4+, alkali metal cations and BaS are soluble.
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides, Ba(OH)2and Sr(OH)2 are soluble in water.
Answer to Problem 24PS
Water soluble compound. Produced ions are.Pb2+and NO−3.
Explanation of Solution
The given compound is Lead (II) nitrate which is soluble in water.
Pb(NO3)2 is dissolves in water, Lead(II) nitrate is composed of Pb2+ (aq) + 2 NO-3 (aq), and it became ions in water.
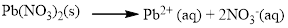
(d)
Interpretation:
Water solubility of the given compounds and ions produced by the compounds should be analyzed.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of (Na+), (K+), (NH4+), (NO3-), (ClO3-), (ClO4-)and(CH3CO2-) are soluble.
Almost all the salts of Cl-, Br-, I-(halides) are soluble. But some of the halides (Ag+, Hg22+, Pb2+) are insoluble.
Salts of F- are soluble. But some of the fluoride salt of Mg2+, Ca2+, Sr2+, Ba2+, Pb2+ are insoluble.
Salts of sulfate (SO42-) are soluble. But sulfates of Ca2+, Sr2+, Ba2+, Pb2+, Ag+, are insoluble.
Insoluble compounds in water:
Most of the salts of (CO32-), (PO43-), (C2O42-), (CrO42-) and(S2-) are insoluble but some of the salts of NH4+, alkali metal cations and BaS are soluble.
Most of the metal hydroxides and oxides are insoluble in water bit some of the alkali metal hydroxides, Ba(OH)2and Sr(OH)2 are soluble in water.
Answer to Problem 24PS
This compound insoluble in water.
Explanation of Solution
The given compound is Barium sulfate which is insoluble in water.
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- 2. Identify the reagents you would need to achieve the following. You may need to consider using a protecting group. HO 1. 2. 3. 4. 5. OH Br HOarrow_forwardBeF2 exists as a linear molecule. Which kind of hybrid orbitals does Be use in this compound? Use Orbital Diagrams to show how the orbitals are formed. (6)arrow_forwardPlease answer the questions and provide detailed explanations as well as a drawing to show the signals in the molecule.arrow_forward
- Propose an efficient synthesis for the following transformation: EN The transformation above can be performed with some reagent or combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. t-BuOK B. Na2Cr2O7, H2SO4, H2O C. NBS, heat F. NaCN D. MeOH E. NaOH G. MeONa H. H2O I. 1) O3; 2) DMSarrow_forwardStereochemistry Identifying the enantiomer of a simple organic molecule 1/5 Check the box under each structure in the table that is an enantiomer of the molecule shown below. If none of them are, check the none of t above box under the table. Br ま HO H 0 Molecule 1 Molecule 2 Molecule 3 OH H Br H H" Br OH Br Molecule 4 Br H OH + + OH Molecule 5 Br H OH none of the above Molecule 6 Br H... OHarrow_forwardPlease answer the questions and provide detailed explanations.arrow_forward
- Question 16 0/1 pts Choose the correct option for the following cycloaddition reaction. C CF3 CF3 CF3 CF3 The reaction is suprafacial/surafacial and forbidden The reaction is antarafacial/antarafacial and forbidden The reaction is antarafacial/antarafacial and allowed The reaction is suprafacial/surafacial and allowedarrow_forward1. Give the structures of the products obtained when the following are heated. Include stereochemistry where relevant. A NO2 + NO2 B + C N=C CEN + { 2. Which compounds would you heat together in order to synthesize the following?arrow_forwardExplain how myo-inositol is different from D-chiro-inositol. use scholarly sources and please hyperlink.arrow_forward
- What is the molarisuty of a 0.396 m glucose solution if its density is 1.16 g/mL? MM glucose 180.2 /mol.arrow_forwardProvide the proper IUPAC or common name for the following compound. Dashes, commas, and spaces must be used correctly. Br ......Im OHarrow_forwardCan you please help me solve this problems. The top one is just drawing out the skeletal correct and then the bottom one is just very confusing to me and its quite small in the images. Can you enlarge it and explain it to me please. Thank You much (ME EX1) Prblm #33arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



