
Custom eBook for Organic Chemistry
2nd Edition
ISBN: 9798214171104
Author: Straumanis
Publisher: Cengage Custom
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3, Problem 1CTQ
Interpretation Introduction
Interpretation:Maximum number of electrons that can fit in a single orbital either should be determined.
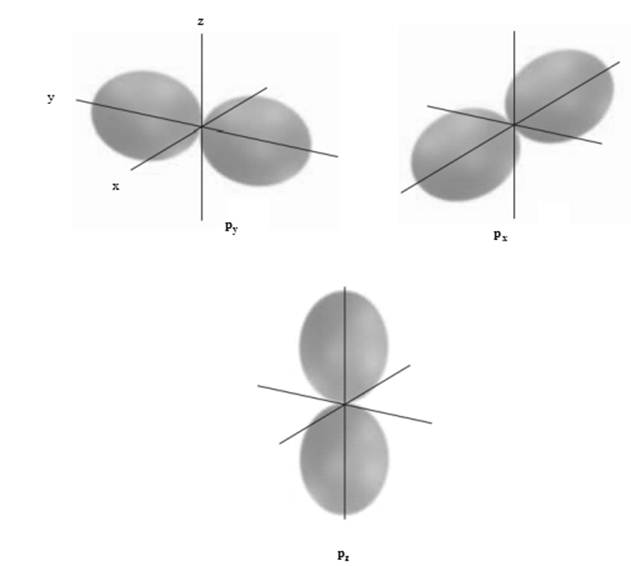
Concept introduction:The shape of three p-orbital is illustrated as follows:
Expert Solution & Answer
Answer to Problem 1CTQ
Two electrons are maximum occupancy of each of the orbitals
Explanation of Solution
The second shell has four orbitals namely
Where,
Inthe second shell, maximum electrons to be accommodated are
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
True or false?
The 4d orbital does not exist in the carbon atom.
Justify your answer in 1 sentence or 2.
What is the energy (in J) of an electron in an He" ion excited to an orbit with n=5? Express your answer in scientific notation, correct to 3 decimal places.
Type your answer.....
A triply ionized beryllium ion, Be3+Be3+ (a beryllium atom with three electrons removed), behaves very much like a hydrogen atom, except that the nuclear charge is four times as great.
A) What is the ground-level energy of Be3+Be3+?
Express your answer in electronvolts to three significant figures.
C) What is the ionization energy of Be3+Be3+?
Express your answer in electronvolts to three significant figures.
E) For the hydrogen atom, the wavelength of the photon emitted in the nn = 2 to nn = 1 transition is 122 nmnm . What is the wavelength of the photon emitted when a Be3+Be3+ ion undergoes this transition?
Express your answer in nanometers to three significant figures.
F) For a given value of nn, how does the radius of an orbit in Be3+Be3+ compare with that for hydrogen?
For a given value of , how does the radius of an orbit in compare with that for hydrogen?
a) The radius of Be3+Be3+ is equal to the hydrogen atom value.
b) The radius of Be3+Be3+ is 1414 times the…
Chapter 3 Solutions
Custom eBook for Organic Chemistry
Ch. 3 - Prob. 1CTQCh. 3 - What neutral atom is represented by the electron...Ch. 3 - Prob. 3CTQCh. 3 - Consider any one of the four identical hybrid...Ch. 3 - Prob. 5CTQCh. 3 - Prob. 6CTQCh. 3 - Prob. 7CTQCh. 3 - Prob. 8CTQCh. 3 - Prob. 9CTQCh. 3 - Prob. 10CTQ
Ch. 3 - On the left side of Figure 3.6, label the areas...Ch. 3 - Prob. 12CTQCh. 3 - Prob. 13CTQCh. 3 - Prob. 14CTQCh. 3 - Prob. 15CTQCh. 3 - Now consider the fully formed molecule on the...Ch. 3 - Prob. 1ECh. 3 - Explain why the two molecules below cannot...Ch. 3 - Prob. 3ECh. 3 - Consider the incomplete orbital representation of...Ch. 3 - Consider the following orbital representation of...Ch. 3 - Summarize how one determines the hybridization...Ch. 3 - Explain what is wrong with each of the following...Ch. 3 - Prob. 8ECh. 3 - Prob. 9ECh. 3 - Complete the following tables, and memorize their...Ch. 3 - Draw orbital representations of bonding in water...Ch. 3 - Draw electron configuration diagrams for carbon in...Ch. 3 - Prob. 13E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using Fig. 2-30, list the elements (ignore the lanthanides and actinides) that have ground-state electron configurations that differ from those we would expect from their positions in the periodic table.arrow_forwardEstimate the probability of finding an electron which is excited into the 2s orbital of the H atom, looking in a cubical box of volume 0.751036m3 centered at the nucleus. Then estimate the probability of finding the electron if you move the volume searched to a distance of 105.8 pm from the nucleus in the positive z direction. (Note that since these volumes are small, it does not matter whether the volume searched is cubical or spherical.)arrow_forwardWhat is the maximum number of electrons that can occupy a f subshell (l = 3)?arrow_forward
- • identify an orbital (as 1s, 3p, etc.) from its quantum numbers, or vice versa.arrow_forward(a) Use the radial wave function for the 3p orbital of a hydrogen atom (see Table 5.2) to calculate the value of r for which a node exists. (b) Find the values of r for which nodes exist for the 3s wave function of the hydrogen atom.arrow_forwardWhat experimental evidence supports the quantum theory of light? Explain the wave-particle duality of all matter .. For what size particles must one consider both the wave and the particle properties?arrow_forward
- Which of the following is a valid set of quantum numbers for an electron in a hydrogen atom? (a) n = 1, = 0, m = 0, ms = 1 (b) n = 1, = 1, m = 0, ms = 1/2 (c) n = 1, = 0, m = 1, ms = + 1/2 (d) n = 1, = 0, m = 0, ms = 1/2arrow_forwardI'm reviewing for my final. This is an old problem that I got correct (option a), but I can't remember how. I know that SeCl2 is AX2E2, which is sp3, but I'm stuck trying to figure out how I chose option a and not option b. How do I know the difference in the amount of arrows (e-) in the p orbital boxes? Thanks :)arrow_forwardGive only typing answer with explanation and conclusion 1. How many degenerate orbitals are in each of the following subshells? (Enter 0 if there are none.) a) n = 6, l = 0 2. Identify the subshell in which electrons with the following quantum numbers are found. (Enter your answers in the format 1s, 2p, etc.) a) n = 6, l = 4 b) n = 2, l = 0 c) n = 4, l = 3arrow_forward
- (Q1). Out of these atoms (S,N,Mn, Ni) determine which one has the most unpaired electrons in its ground state electron configuration.arrow_forwardAnswer the following: 1) Calculate the energy of an electron in the first 5 energy levels (1-5) of a hydrogen atom. 2)Sketch the relative energy levels 1-5 based on your values from #1. Compare the different from 1-2 with 2-3, 3-4, and 4-5.arrow_forward0,1,2,3,4 If n 5, what are the possible values of (? If (- 1, what are the possible values of m, ? If there is more than one possible value, type them as a list For example, if an answer could be 0, 1, or 2, you would type in "0, 1, 2". (Canvas will be very picky about this, but l'll look them over when I grade it manually and I'll give credit if your values were correct.) Question 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning