What is quantum theory?
Quantum theory, often known as quantum physics, is the foundation of modern physics and encompasses the nature and behavior of matter and energy at the atomic and molecular levels. As the exact nature and behavior of atomic and subatomic particles are impossible to determine, the laws of classical physics do not apply to this discipline. As a result, quantum field theory is primarily based on assumptions and probabilities.
Development of quantum mechanics
Classical physics might answer the questions about how to push a car or throw a ball, at the macroscopic level but not at the subatomic level. The nature and behavior of microscopic objects such as atoms and molecules necessitated a new set of principles, which led to the development of quantum physics. In the 1990s, scientist Max Plank offered one of the earliest concepts that laid the foundation for quantum mechanics. Based on his black body radiation experiment (a hypothetical body that completely absorbs all the radiant energy falling upon it; and as it reaches a temperature equilibrium, it bounces back all of the absorbed energy quickly), he saw that the blackbody did not emit unlimited energy as classical physics expected. This led him to believe that energy was made up of discrete units known as quanta, rather than a continuous electromagnetic wave.
In 1905, Albert Einstein demonstrated in his photoelectric experiment that when light is shone on a metal surface, electrons from the ground state turn to an excited state and get ejected from the sample based on the frequency of the light rather than the intensity. He also claimed that photons were the particles that made up electromagnetic radiation. The quantum energy of each of these photons is equal to the frequency required for the electron emission (stimulated emission). This led scientists to believe that the classical physics wave theory of electromagnetic radiation was insufficient to explain such behavior, and this led them to believe that light exhibits wave-particle duality.

CC BY-SA 4.0 | Image credits: https://commons.wikimedia.org/wiki | MikeRun
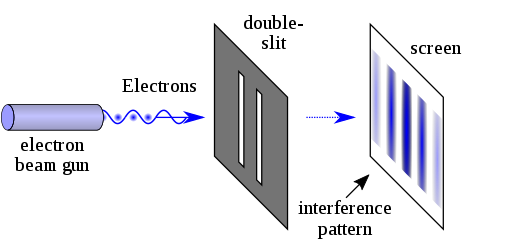
G.P. Thompson devised the double-slit experiment to demonstrate light's wave-particle duality. He generated a thin band by directing a stream of electrons from an x-ray at a metal foil through a tiny slit. Similarly, he replicated the experiment with the electron beam traveling through two slits but instead of two bands, he saw an interface pattern similar to that of a wave. This demonstrated that light may behave both as a wave and as a particle (photon).
In 1924, Louis de Broglie demonstrated that matter as light may behave as a wave and display wave-particle duality. He demonstrated this by showing that electrons as light, can be diffracted. In addition to particle qualities, this resulted in wave function properties for electrons.
In 1927, Heisenberg developed the uncertainty principle, which is based on matter's wave-particle duality. He claimed that measuring and calculating an object's position and momentum is impossible. When an electron with a mass of 9.109 383 7015 x 10-31 kg collides with a bright light source, it is illuminated. This illumination helps in the identification of its position. However, the impact causes an increase in its momentum resulting in an error in its momentum measurement. As a result, at any instant in time, either position or momentum can be precisely measured. However, in the macroscopic universe, which is governed by the so-called Newtonian rules, it is possible to determine both the position and the momentum of massive objects at a given time. This demonstrated that, unlike classical physics, which stipulates that a particle is a particle and a wave is a wave, quantization principles do not follow this rule. The electrons in atoms and the photons in light are not necessarily particles or waves, although they do have properties of both.
Interpretations of quantum theory
The Copenhagen interpretation and the many-world theory
There are two interpretations of quantum mechanics- Copenhagen interpretation, and many-worlds theory. It was Niels Bohr who illustrated the Copenhagen interpretation. His interpretation asserts that a particle cannot be presumed to have certain attributes until it is measured; until then, it can exist as a particle or as a wave. In a nutshell, it implies that, until a quantum system is measured, it exists in numerous states at the same time. This idea is known as superposition. The theory can be further demonstrated with the help of Schrödinger's Cat. A living cat is placed in a self-contained lead box in this hypothetical idea. The cat is alive at this point, but when a vial of cyanide is tossed into the box and sealed, we would not know whether it is alive or dead. It can be alive if the cyanide vial is not shattered, or it could be dead if the vial is shattered. We will know if the cat is alive or dead only when we open the box.
Many-worlds or multiverse theory, according to the second interpretation, is a hypothetical group of several universes that exist in parallel at the same time and space as our universe. Richard Feynman (one of the founders of modern quantum mechanics) and Stephen Hawking, two well-known physicists, both believed in this idea.
Practical applications of quantum theory
The principles of quantum mechanics find applications in a lot of areas such as:
Quantum optics
A field of optical physics dealing with the interaction of photons with atoms and molecules using the principles of quantum mechanics.
Quantum computing
It involves the applications of quantum theory features such as superposition to perform computations on quantum computers.
Quantum chemistry
A discipline of chemistry that studies chemical systems, using quantum mechanics. As opposed to classical computing that encodes information in bits, quantum computing encodes information in qubits.
Quantum cryptography
A subset of cryptography that involves the applications of quantum-mechanical phenomena to fulfill cryptographic tasks for secure communications
Superconducting magnets
Superconducting magnets are electromagnets built of superconducting wire that produce stronger magnetic fields devoid of electrical resistance using techniques of quantum theory. Their electrodynamics find applications in several techniques such as NMR.
Semiconductors
Modern semiconductors, such as light-emitting diodes, optical amplifiers, and transistors, rely on energy gaps that follow the wave-like behavior of electrons (quantum theory) to display their superconductivity.
Imaging techniques such as MRI and electron microscopy
The MRI (magnetic resonance imaging) approach is based on NMR (nuclear magnetic resonance) technology, which generates images of the bodily organs, using a combination of powerful magnetic fields and radio waves. This method differs from other medical imaging methods such as CT (computed tomography) scans in that it does not require the applications of x-ray. Electron microscopy uses accelerated electron beams to illuminate an object with high resolution, and it finds applications in the study of microorganisms.
Atomic clocks
They are the most precise timekeeping devices that rely on interactions between electromagnetic radiations and the energy levels of specific atoms by the principles of quantum mechanics. Atomic clocks find applications in TV broadcasts and GPS navigation systems as the primary time and frequency standard.
Context and Applications
This subject is important in professional exams for both undergraduate and graduate levels such as
- Masters in Science in Quantum Physics
- Masters in Science in Nuclear Physics
- Masters in Science in Physical Chemistry
- Masters in Science in Organic and Inorganic Chemistry
- Masters in Science in Mathematics
- Bachelors in Science in Mathematics
Practice Problems
Question1) Which theory is primarily based on assumptions and probabilities?
- Quantum field
- Physics
- Mathematics
- Classical
Answer- Option a
Explanation- It is impossible to identify the exact nature and behavior of atomic and subatomic particles; so, quantum field theory is based mostly on assumptions and probabilities.
Question 2) A black body is a hypothetical body that _____ absorbs all the radiant energy falling upon it.
- Partially
- Completely
- Nil
- Half
Answer- Option b
Explanation- A black body is a hypothetical physical body that absorbs all the incoming electromagnetic radiation, irrespective of frequency.
Question 3) What is the mass of an electron?
- 0
- 9.109 383 7015 x 10
- 9.109 383 7015 x 10-31 kg
- 9.109 383 7015
Answer- Option c
Explanation- The mass of an electron is 9.109 383 7015 x 10-31 kg and is considered as negligible in comparison to mass of a proton or neutron, hence mass of an electron is not considered when calculating the mass number of an atom.
Question 4) What is the full form of MRI?
- Magnetic resonance imaging
- Resonance imaging
- Nuclear magnetic resonance
- Magnetic resonance
Answer- Option a
Question 5) In which unit does the quantum computing encode information?
- Bits
- Twits
- Qubit
- Dots
Answer- Option c
Explanation- A qubit (or quantum bit) is the basic unit of quantum computing similar to that of a bit in classical computing.
Common mistakes
The most common mistake students make is mixing classical physics concepts with mathematical concepts of quantum mechanics.
Related Concepts
- Uncertainty principle
- Many-body theory
- Solid-state quantum computing
- Quantum information
- Quantum states
- Quantum formalism
Want more help with your chemistry homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
Techniques and applications of quantum theory Homework Questions from Fellow Students
Browse our recently answered Techniques and applications of quantum theory homework questions.