
(a)
Interpretation:
A mechanism should be proposed for the given reaction.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”
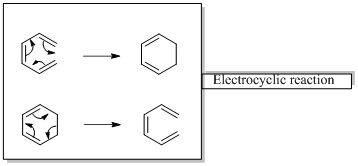
Diels-alder reaction is a cycloaddition reaction which is occurs between a conjugated diene and substituted
Mechanism gives the step wise processes occurs in a particular reaction.
(b)
Interpretation:
The product should be determined for the given reaction if trans-2-butene were used instead of ethene.
Concept introduction:
Pericyclic reactions are “ any concerted reaction in which bonds are formed or brocken in a cyclic transition state”. There is a single transition state from start to finish, in contrast to a stepwise reaction.
In an electrocyclic reaction “one new sigma- bond is formed or brocken.”

Diels-alder reaction is a cycloaddition reaction which is occurs between a conjugated diene and substituted alkene to form cyclohexene ring system. This concerted reaction can be accelerated by heating or using some catalysts.
Mechanism gives the step wise processes occurs in a particular reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 28 Solutions
Organic Chemistry, Books a la Carte Edition (8th Edition)
- The diene shown below will NOT react in a Diels-Alder reaction. Why not? Select one: O A. The compound is lacking in electron donating groups. B. The compound is not a conjugated diene. OC. The compound is lacking in electron withdrawing groups. OD. This compound cannot adopt the s-cis conformation.arrow_forwardIn the alkene below, why is HB more easily abstracted by a halogen atom than HẠ ? НА HB A. Abstraction of Hg forms a carbocation, while abstraction of HA forms an carbanion. B. Abstraction of HB produces a more stable free radical. C. HB is abstracted as a stable H* ion, but HA is not. D. Abstraction of HB involves less steric hindrance.arrow_forwardThe diene shown below will NOT react in a Diels-Alder reaction. Why not? Select one: A. The compound is not a conjugated diene. B. The compound is lacking in electron withdrawing groups. O C. This compound cannot adopt the s-cis conformation. D. The compound is lacking in electron donating groups.arrow_forward
- Which is the MAJOR product of the following reaction? Et 1) BH3:THF 2) H2O2, NaOH Which of the following best describes a key step in the mechanism for the reaction below? HO ... CH3 -CH3 dihydroxylation + en H3C- H3C- HO. electrophilic addition reaction to form a carbocation intermediate B nucleophilic attack by an alkene to form a cyclic (epoxide) intermediate elimination reaction by abstraction of a beta-hydrogen D free-radical substitution at the carbonyl carbon Which alkene will produce the HIGHEST yield of the alkyl halide below? Br. alkene HBr |arrow_forwardWhich set of reagents is used for the Markovnikov addition of water to an alkene without rearrangement? A. BH3, THF followed by H₂O2, NaOH B. H₂O, H₂SO4 C. Hg(O₂CCH3)2, H₂O followed by NaBH4, NaOH D. none of thesearrow_forwarda. How many alkenes could you treat with H2, Pd/C to prepare methylcyclopentane? b. Which of the alkenes is the most stable? c. Which of the alkenes has the smallest heat of hydrogenation?arrow_forward
- 1. Circle the aromatic molecules NOTE: bicyclic compounds with just one aromatic ring are still considered aromatic molecules 2. Provide the major organic product of the following Diels-Alder Reaction. NCarrow_forward2. Consijder the reactiojn scheme below a. Draw the major product of this reaction. Show clearly (using wedge-and-dash lines) all stereoisomers formed under these reaction conditions. 1. BH3:THF 2. H2O2, NaOH b. What is the stereochemical relationship between the different stereoisomer products you drew in part 2a.?arrow_forwardA. Predict the expected product(s) of the EAS reactions shown below (assume that substitution occurs only once). NO ₂ Br₂, FeBr3 HNO3 H₂SO4 conc. H₂SO4arrow_forward
- a. What is the major monobromination product of the following reaction? Disregard stereoisomers. b. What is the anticipated percent yield of the major product (as a percentage of all the monobrominated products)?arrow_forward19. Predict the major product for the following reaction. 22. Predict the major product for the following Diels-Alder reaction.arrow_forwardWhich stereoisomer would be produced from the reaction of trans-2-butene with OsO4 followed by H2O2? A. 2S,3S- diol and 2R, 3R-diol B. 2S,3R-diol and 2R,3S-diol C. 2S,3S-diol only D. 2R, 3R-diol only E. 2S,3R-diol onlyarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
