
a)
Interpretation:
The products need to be predicted when tripalmitolein is treated with the given set of reagents.
Concept introduction:
Reduction is a reaction in which hydrogen is added to the given compound. One of the examples is conversion of unsaturated bonds to saturated bonds.
To predict: the product formed when tripalmitolein is treated with excess hydrogen and nickel.
a)
Answer to Problem 29PP
Solution:
The product predicted when tripalmitolein is treated with hydrogen and nickel is,
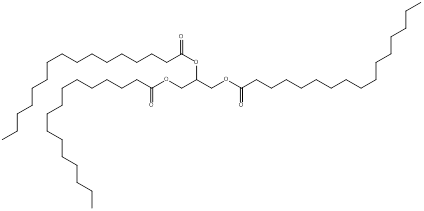
Explanation of Solution
Structure of tripalmitolein
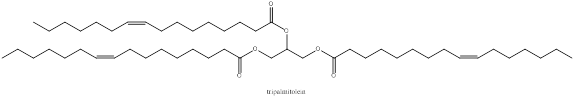
The structure of tripalmitolein is drawn as above. In tripalmitolein there are three double bonds.
Reaction with excess hydrogen and nickel.
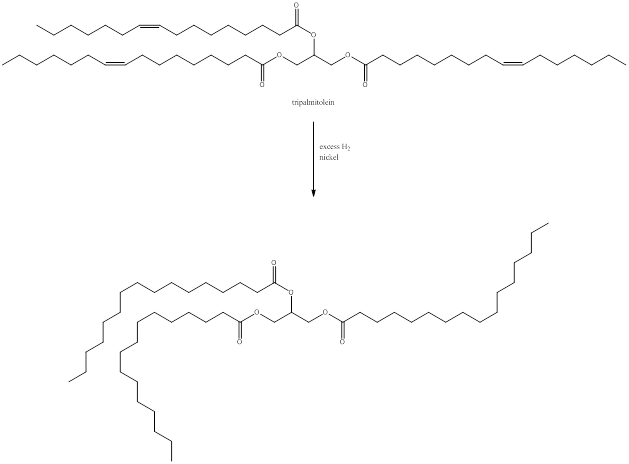
When tripalmitolein is treated with excess hydrogen and nickel, all the double bonds present in the fatty acid residue are reduced to saturated bonds to give a saturated triglyceride as shown above.
The product formed when tripalmitolein undergoes reaction with excess hydrogen and nickel is a saturated triglyceride and the structure is,
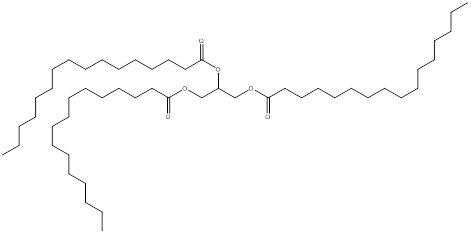
(b)
Interpretation:
The products need to be predicted when tripalmitolein is treated with the given set of reagents.
Concept introduction:
When triglyceride is treated with aqueous sodium hydroxide, the ester group is hydrolyzed to give glycerol and the fatty acid as carboxylate ion.
To predict: the product formed when tripalmitolein is treated with aqueous sodium hydroxide.
(b)
Answer to Problem 29PP
Answer
The product obtained when tripalmitolein is treated with aqueous base is,
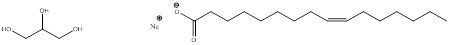
Explanation of Solution
Structure of tripalmitolein

The structure of tripalmitolein is drawn as above. In tripalmitolein there are three ester groups.
Reaction with excess hydrogen and nickel.
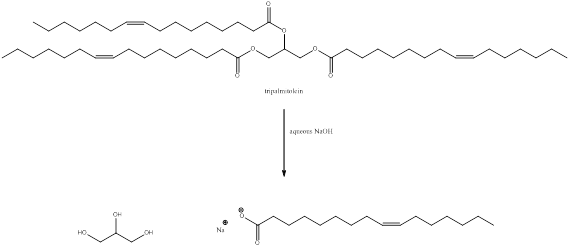
When tripalmitolein is treated with aqueous sodium hydroxide the ester group is hydrolyzed to form glycerol and fatty acid carboxylate ion.
The product formed when tripalmitolein is treated with aqueous sodium hydroxide is glycerol and three equivalents of carboxylate ion,

Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





