
a)
Interpretation:
The products need to be predicted when tripalmitolein is treated with the given set of reagents.
Concept introduction:
Reduction is a reaction in which hydrogen is added to the given compound. One of the examples is conversion of unsaturated bonds to saturated bonds.
To predict: the product formed when tripalmitolein is treated with excess hydrogen and nickel.
a)
Answer to Problem 29PP
Solution:
The product predicted when tripalmitolein is treated with hydrogen and nickel is,
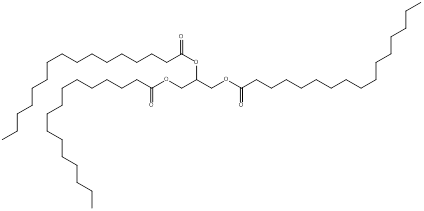
Explanation of Solution
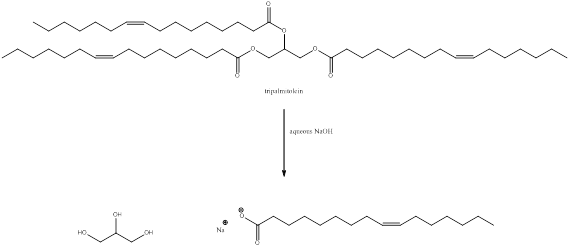
Structure of tripalmitolein
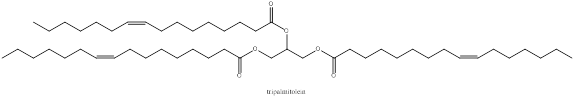
The structure of tripalmitolein is drawn as above. In tripalmitolein there are three double bonds.
Reaction with excess hydrogen and nickel.
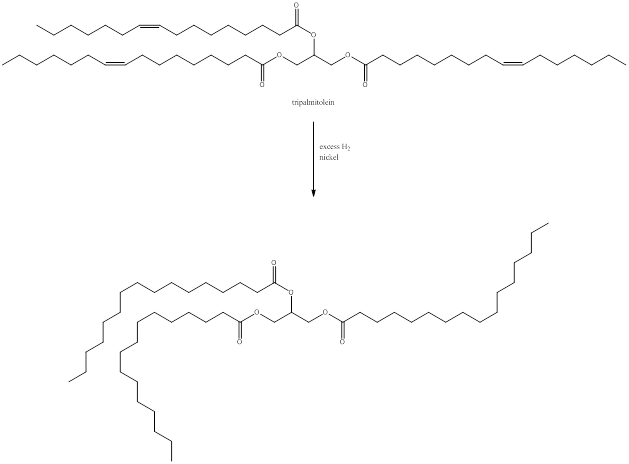
When tripalmitolein is treated with excess hydrogen and nickel, all the double bonds present in the fatty acid residue are reduced to saturated bonds to give a saturated triglyceride as shown above.
The product formed when tripalmitolein undergoes reaction with excess hydrogen and nickel is a saturated triglyceride and the structure is,
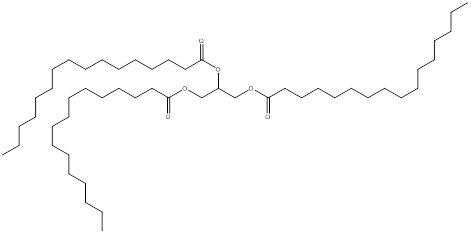
(b)
Interpretation:
The products need to be predicted when tripalmitolein is treated with the given set of reagents.
Concept introduction:
When triglyceride is treated with aqueous sodium hydroxide, the ester group is hydrolyzed to give glycerol and the fatty acid as carboxylate ion.
To predict: the product formed when tripalmitolein is treated with aqueous sodium hydroxide.
(b)
Answer to Problem 29PP
Answer
The product obtained when tripalmitolein is treated with aqueous base is,
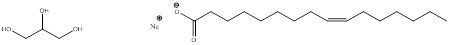
Explanation of Solution
Structure of tripalmitolein

The structure of tripalmitolein is drawn as above. In tripalmitolein there are three ester groups.
Reaction with excess hydrogen and nickel.
When tripalmitolein is treated with aqueous sodium hydroxide the ester group is hydrolyzed to form glycerol and fatty acid carboxylate ion.
The product formed when tripalmitolein is treated with aqueous sodium hydroxide is glycerol and three equivalents of carboxylate ion,

Want to see more full solutions like this?
Chapter 26 Solutions
ORG. CHEM. TEXT/STUDY GUIDE/EBOOK (LL)
- 4. Propose a synthesis of the target molecules from the respective starting materials. a) b) LUCH C Br OHarrow_forwardThe following mechanism for the gas phase reaction of H2 and ICI that is consistent with the observed rate law is: step 1 step 2 slow: H2(g) +ICI(g) → HCl(g) + HI(g) fast: ICI(g) + HI(g) → HCl(g) + |2(g) (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. + → + (2) Which species acts as a catalyst? Enter formula. If none, leave box blank: (3) Which species acts as a reaction intermediate? Enter formula. If none, leave box blank: (4) Complete the rate law for the overall reaction that is consistent with this mechanism. (Use the form k[A][B]"..., where '1' is understood (so don't write it) for m, n etc.) Rate =arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forward
- 1. For each of the following statements, indicate whether they are true of false. ⚫ the terms primary, secondary and tertiary have different meanings when applied to amines than they do when applied to alcohols. • a tertiary amine is one that is bonded to a tertiary carbon atom (one with three C atoms bonded to it). • simple five-membered heteroaromatic compounds (e.g. pyrrole) are typically more electron rich than benzene. ⚫ simple six-membered heteroaromatic compounds (e.g. pyridine) are typically more electron rich than benzene. • pyrrole is very weakly basic because protonation anywhere on the ring disrupts the aromaticity. • thiophene is more reactive than benzene toward electrophilic aromatic substitution. • pyridine is more reactive than nitrobenzene toward electrophilic aromatic substitution. • the lone pair on the nitrogen atom of pyridine is part of the pi system.arrow_forwardThe following reactions are NOT ordered in the way in which they occur. Reaction 1 PhO-OPh Reaction 2 Ph-O -CH₂ heat 2 *OPh Pho -CH2 Reaction 3 Ph-O ⚫OPh + -CH₂ Reaction 4 Pho Pho + H₂C OPh + CHOPh H₂C -CH₂ Reactions 1 and 3 Reaction 2 O Reaction 3 ○ Reactions 3 and 4 ○ Reactions 1 and 2 Reaction 4 ○ Reaction 1arrow_forwardSelect all possible products from the following reaction: NaOH H₂O a) b) ОН HO O HO HO e) ОН f) O HO g) h) + OHarrow_forward
- 3. Draw diagrams to represent the conjugation in these molecules. Draw two types of diagram: a. Show curly arrows linking at least two different ways of representing the molecule b. Indicate with dotted lines and partial charges (where necessary) the partial double bond (and charge) distribution H₂N* H₂N -NH2arrow_forwardQuestion 2 of 25 point Question Attempt 3 of Ulimited Draw the structure for 3-chloro-4-ethylheptane. Part 2 of 3 Click and drag to start drawing a structure. Draw the structure for 1-chloro-4-ethyl-3-lodooctane. Click and drag to start drawing a structure. X G X B c Part 3 of 30 Draw the structure for (R)-2-chlorobutane. Include the stereochemistry at all stereogenic centers. Check Click and drag to start drawing a structure. G X A 。 MacBook Pro G P Save For Later Submit Assignment Privacyarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- In a silicon and aluminum alloy, with 12.6% silicon, what are the approximate percentages of the phases present in the constituent that is formed at the end of solidification? Temperature (°C) 1500 1000 L B+L 1415- α+L 577' 500 1.65 12.6 99.83 α+B B 0 Al 20 40 60 Weight percent silicon 80 Siarrow_forwardPlease correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





