
(a)
Interpretation:
The structure of
Concept introduction:
The systematic naming of organic compound is given by
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
- In the heterocyclic compound, azo for nitrogen, oxo for oxygen, thio for sulfur and so on is used.
Answer to Problem 26.1P
The structure of the given compound,
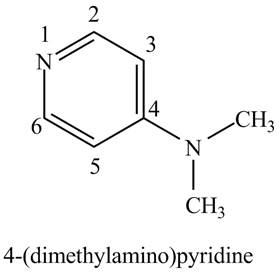
Explanation of Solution
The name of the given compound
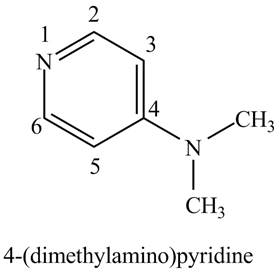
Figure 1
The structure of the given compound,
(b)
Interpretation:
The structure of
Concept introduction:
The systematic naming of organic compound is given by IUPAC nomenclature. The naming of organic compound is done such that the structure of the organic compound is correctly interpreted from the name.
Rules for writing IUPAC name from the structural formula are given below.
- First, identify the longest carbon chain.
- The next step is to identify the groups attached to the longest chain.
- Identify the position, location, and a number of the substituents bonded to the carbon chain.
- Use prefix di, tri, tetra if the same type of substituents is present.
- Name the substituents in alphabetical order.
- In the heterocyclic compound, azo for nitrogen, oxo for oxygen, thio for sulfur and so on is used.
Answer to Problem 26.1P
The structure of the given compound,
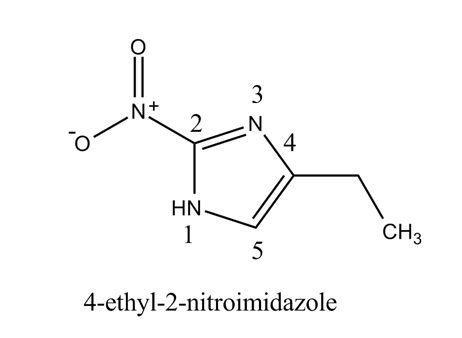
Explanation of Solution
The given compound

Figure 2
The structure of the given compound,
Want to see more full solutions like this?
Chapter 26 Solutions
EBK ORGANIC CHEMISTRY
- 5. Give the structural formulae and name the functional groups of the following compounds. (a) 3-chlorobut-1-ene (b) butanedioic acid Name the functional group: (c) propanamide Name the functional group: (d) 3-methylbutanal Name the functional group: Name the functional group:arrow_forwardGive the chemical tests to distinguish between the following pairs of compounds :(i) Ethyl amine and Aniline(ii) Aniline and Benzylaminearrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?(c) The heat of hydrogenation for biphenyl is about 418 kJ>mol (100 kcal>mol). Calculate theresonance energy of biphenyl.(d) Compare the resonance energy of biphenyl with that of naphthalene and with that of two benzene rings. Explain thedifference in the resonance energies of naphthalene and biphenyl.arrow_forward
- (a) Compound Z is a tertiary aromatic amine with the formula, C8H11N. Provide a chemical structure for compound Z. (b)nDraw the structure of the product formed exclusively when nitrous acid reacts with Z.arrow_forwardDraw the structures of the following compounds:(a) tert-butylaminearrow_forwardIn each of the following reactions, two possible organic products can be formed. Draw both organic products in each case and then circle the one formed in greatest quantity in each case. HC (a) 1) NaH, 2) acid (b) CH,CH,OH (c) CH,CH,OH NH2 (d) Oarrow_forward
- Draw structural formulas for these compounds. (a) 4-Phenyl-1-pentene (b) p-Cresol (c) 2,4-Dichlorophenolarrow_forwardDraw structures corresponding to these names:(a) 4-Methylpentanamide (b) N-Ethyl-N-methylpropanamidearrow_forwardGive a chemical test to distinguish between each of the following pairs of compounds :(i) Ethylamine and Aniline(ii) Aniline and Benzylaminearrow_forward
- Imidazole boils at 257 °C, whereas N-methylimidazole boils at 199 °C. Explain the difference in boiling points.arrow_forward34) The product formed by the reaction of toluene with chlorine in the presence of sunlight is: (a) o-chlorotoluene (b) 2,4-dichlorotoluene (c) p-chlorotoluene (d) Benzylchloridearrow_forwardName and draw a structural formula for the major product of each alkene addition reaction: CH S (a) CH₂C=CH₂ + HI →→→→ (b) CH 3 + HClarrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
