
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 25.5, Problem 25.13P
(a) Which compound, morphine or heroin, is more polar? (b) Which compound can cross the blood-brain barrier more readily? (c) Explain why heroin is a more potent pain reliever than morphine. 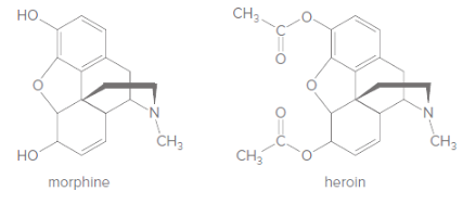
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Viscosity of a liquid related to the activation energy.
Vibrational contributions to internal energy and heat capacity1) are temperature independent2) are temperature dependent
The approximation of calculating the partition function by integration instead of the summation of all the energy terms can only be done if the separation of the energy levels is much smaller than the product kT. Explain why.
Chapter 25 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 25.1 - Prob. 25.1PCh. 25.1 - Prob. 25.2PCh. 25.2 - Compare erythrocytes, leukocytes, and platelets...Ch. 25.2 - Prob. 25.4PCh. 25.2 - Prob. 25.5PCh. 25.2 - Prob. 25.6PCh. 25.2 - Prob. 25.7PCh. 25.3 - Prob. 25.8PCh. 25.3 - Prob. 25.1PPCh. 25.3 - Prob. 25.9P
Ch. 25.3 - Prob. 25.10PCh. 25.4 - Prob. 25.11PCh. 25.4 - Prob. 25.12PCh. 25.5 - (a) Which compound, morphine or heroin, is more...Ch. 25.5 - Prob. 25.14PCh. 25.6 - Prob. 25.15PCh. 25.6 - Prob. 25.16PCh. 25.6 - Prob. 25.17PCh. 25.6 - Individuals with a rare condition called diabetes...Ch. 25 - Prob. 19PCh. 25 - Prob. 20PCh. 25 - Prob. 21PCh. 25 - Prob. 22PCh. 25 - Prob. 23PCh. 25 - Prob. 24PCh. 25 - Prob. 25PCh. 25 - Prob. 26PCh. 25 - For each substance, indicate if it is present in...Ch. 25 - Prob. 28PCh. 25 - Prob. 29PCh. 25 - Prob. 30PCh. 25 - Prob. 31PCh. 25 - Prob. 32PCh. 25 - Prob. 33PCh. 25 - Prob. 34PCh. 25 - Prob. 35PCh. 25 - Which of the following locations corresponds to...Ch. 25 - Prob. 37PCh. 25 - Prob. 38PCh. 25 - Prob. 39PCh. 25 - Prob. 40PCh. 25 - Prob. 41PCh. 25 - Prob. 42PCh. 25 - Explain how abnormally fast respirationthat is,...Ch. 25 - Prob. 44PCh. 25 - Prob. 45PCh. 25 - Prob. 46PCh. 25 - Prob. 47PCh. 25 - Prob. 48PCh. 25 - When blood flows through the glomerulus, which...Ch. 25 - Prob. 50PCh. 25 - Prob. 51PCh. 25 - Prob. 52P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain the meaning of: the electron partition function is equal to the degeneracy of the ground state.arrow_forward28. For each of the following species, add charges wherever required to give a complete, correct Lewis structure. All bonds and nonbonded valence electrons are shown. a. b. H H H H H :0-C-H H H H-C-H C. H H d. H-N-0: e. H H-O H-O H B=0 f. H—Ö—Ñ—Ö—H Norton Private Barrow_forwardAt 0oC and 1 atm, the viscosity of hydrogen (gas) is 8.55x10-5 P. Calculate the viscosity of a gas, if possible, consisting of deuterium. Assume that the molecular sizes are equal.arrow_forward
- Indicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardIndicate the correct option for the velocity distribution function of gas molecules:a) its velocity cannot be measured in any other way due to the small size of the gas moleculesb) it is only used to describe the velocity of particles if their density is very high.c) it describes the probability that a gas particle has a velocity in a given interval of velocitiesd) it describes other magnitudes, such as pressure, energy, etc., but not the velocity of the moleculesarrow_forwardDraw the skeletal structure of the alkane 4-ethyl-2, 2, 5, 5- tetramethylnonane. How many primary, secondary, tertiary, and quantenary carbons does it have?arrow_forward
- Electronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardCalculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY