
(a)
Interpretation:
The role of the process of diffusion in the transport of gasses through the body needs to be explained.
Concept Introduction:
The gasses are transported from the lungs to the whole body and this transfer of gases in the lungs takes place by the process of diffusion. The gasses in the lungs diffuse from their region of high pressure to their region of low pressure and are either taken throughout the body or exhale out depending upon the gas.
(b)
Interpretation:
The role of carbaminohemoglobin in the transport of gases throughout the body needs to be explained.
Concept Introduction:
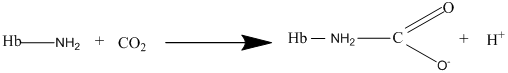
Other than diffusion there are other methods as well for the transport of gases and the carbaminohemoglobin is one of the methods of transport for the carbon dioxide gas where the carbon dioxide bonds with hemoglobin to form carbaminohemoglobin as per the following equation:
(c)
Interpretation:
The role of alveoli in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
Alveoli are the air sacs in the lungs which basically increases the surface area capacity of the lungs and theses very alveoli are the sites of the gases transfer from the atmosphere to the blood in the body.
(d)
Interpretation:
The role of carbonic anhydrase in the transfer of gases throughout the body needs to be explained.
Concept Introduction:
The transfer of carbon dioxide also takes place by the means of carbonic anhydrase. The carbonic anhydrase converts to carbonic acid

Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
General, Organic, and Biological Chemistry - 4th edition
- The liquid portion of the blood is called: a. serum.c. plasma. b. gamma globulin.d. lymph.arrow_forwardWhat term is given to the reversible flow of chloride ions across the red blood cell membrane during the oxygen and carbon dioxide transport process?arrow_forwardHow does the polarity of the phosphoglycerides contribute to their function of forming cell membranes?arrow_forward
- Chemistry 9. Identify the type of transport described by each of the following: a) 0₂ molecules move into the cell from a higher concentration outside the cell. b) H' ions move through a protein channel from high to low concentration.arrow_forwardHow does oxygen travel through the respiratory system?arrow_forwardLipoproteins are globular structures that are responsible for transporting lipids through the blood stream. Two types of lipoproteins are LDL and HDL. Classify each description as applying to LDL, HDL, or both. LDL HDL Both LDL and HDL Answer Bank transport excess cholesterol to the liver transport cholesterol from the liver to other tissues classified by their density "bad" cholesterol "good" cholesterol help regulate lipid metabolism increased levels are associated with atherosclerosis consist of lipids and proteinsarrow_forward
- Explain how your model shows that cellular respiration is exothermic, not endothermic.arrow_forward8 Grades Schoology Schoology S Schoology A bcps.schoology.com/common-assessment-delivery/start/3392434065?actionD E Apps bcps.schoology COURSES GROUPS RESOURCES GRADE REPORT 12/7-Try It: Organ System Introduction Neurons, the spinal cord and brain are all parts of the O digestive system O excretory system O circulatory system O nervous systemarrow_forwardwhat the difference between homeostasis and hemeostasisarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



