
Concept explainers
a)
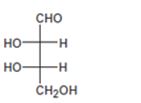
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
In Fischer projections, D sugars have the hydroxyl group on right at the farthest chirality center and L sugars have this hydroxyl group on left.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
b)
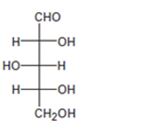
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
c)
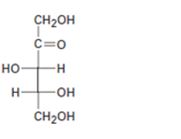
Interpretation:
The configuration as R or S is to be assigned to each chirality center in the monosaccharide given and whether it is a D sugar or L sugar is to be stated.
Concept introduction:
In Fischer projection formula, a tetrahedral carbon is represented by two crossed lines. The horizontal line represents bonds coming out of the page and vertical lines represent bonds moving in to the page.
For assigning R or S configuration, the four groups attached to the chiral center are arranged in the order of priority by applying sequence rules. The molecule is then oriented in such a way that the group of lowest priority points away from the viewer. If the arrangement of highest priority to second highest priority to third highest priority is clockwise then R configuration is assigned. If the arrangement of highest priority to second highest priority to third highest priority is counterclockwise then S configuration is assigned.
To assign:
The configuration as R or S to each chirality center in the monosaccharide given and to state whether it is a D sugar or L sugar.
Trending nowThis is a popular solution!

Chapter 25 Solutions
Organic Chemistry
- Draw a monosaccharide described in the following statements: 1. A diastereomer of D-fructose in the D- configuration. 2. A C-3 epimer of D- glucose. 3. A C-3 epimer of D- fructose.arrow_forwardDraw the linear form and formation ring of the monosaccharide fructose. Draw the linear form and formation ring of monosaccharide glucose. Show the dehydration reaction of the two monosaccharide. Draw the structure of oleic acid Draw the Fischer projection formula of the sugar gulose. Draw the enantiomer of the sugar and indicate the D/L designationarrow_forwardHow is each compound related to the simple sugar D-erythrose? Is it an enantiomer, a diastereomer, or an identical molecule?arrow_forward

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning





