
(a)
Interpretation:
The
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of
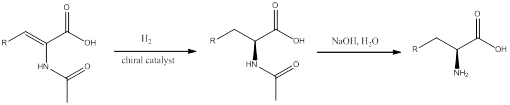
To find: the alkene taken for the synthesis of L-alanine using asymmetric catalytic hydrogenation.
(b)
Interpretation:
The alkenes required for the synthesis of given set of amino acids by asymmetric catalytic hydrogenation need to be identified.
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of amine. The general scheme can be shown as,

To find: the alkene taken for the synthesis of L-alanine using asymmetric catalytic hydrogenation.
(c)
Interpretation:
The alkenes required for the synthesis of given set of amino acids by asymmetric catalytic hydrogenation need to be identified.
Concept introduction:
Asymmetric catalytic hydrogenation is a reaction in which the alkene substrate is reduced to a saturated compound by addition of two atoms of hydrogen. When chiral catalyst is used a enantioselective product is obtained. This technique uses a chiral catalyst so that the amino acids can be prepared with high enantiomeric excess.
The final step after hydrogenation is the hydrolysis of the protected group of amine. The general scheme can be shown as,

To find: the alkene taken for the synthesis of L-tyrosine using asymmetric catalytic hydrogenation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 25 Solutions
ORGANIC CHEMISTRY WILEYPLUS ACCESS>I<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





