
Concept explainers
Practice Problem 24.8
Glutathione is a tripeptide found in most living cells. Partial acid-catalyzed hydrolysis of glutathione yields two dipeptides, CG and one composed of E and C. When this second dipeptide was treated with DNFB, acid hydrolysis gave N-labeled glutamic acid.
(a) On the basis of this information alone, what structures are possible for glutathione?
(b) Synthetic experiments have shown that the second dipeptide has the following structure:
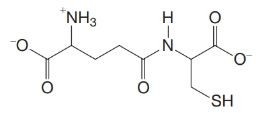
What is the structure of glutathione?
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry & Chemical Reactivity
Basic Chemistry (5th Edition)
Chemistry: The Central Science (13th Edition)
Inorganic Chemistry
Organic Chemistry As a Second Language: Second Semester Topics
General, Organic, and Biological Chemistry (3rd Edition)
- Give the amino acid sequence of an octapeptide that contains the amino acids Tyr, Ala, Leu (2 equiv), Cys, Gly, Glu, and Val, and forms the following fragments when partially hydrolyzed with HCl: Val–Cys–Gly–Glu, Ala–Leu–Tyr, and Tyr–Leu–Val–Cys.arrow_forwardHow many constitutional isomers are possible for a triglyceride containing one molecule each of palmitic acid, oleic acid, and stearic acid? (b) Which of these constitutional isomers are chiral?arrow_forwardDraw zwitterion forms of these amino acids. (a) Valine (b) Phenylalanine (c) Glutaminearrow_forward
- Explain the meaning of the following: (a) Chiral carbon (b) Enantiomers (c) Esterificationarrow_forwardOn complete hydrolysis, a polypeptide gives two alanine, one leucine, one methionine, one phenylalanine, and one valine residue. Partial hydrolysis gives the following fragments: Ala-Phe, Leu-Met, Val-Ala, Phe-Leu. It is known that the first amino acid in the sequence is valine and the last one is methionine. What is the complete sequence of amino acids?arrow_forwardThe dynorphins are a group of opioid peptides that play an importantrole in changes in the brain associated with cocaine addiction. One ofthese peptides, dynorphin A, contains the following amino acidsequence: Tyr–Gly–Gly–Phe–Leu–Arg–Arg–Ile–Arg–Pro–Lys–Leu–Lys.Draw the amino acids and peptide fragments formed when dynorphin A is treated with each reagent or enzyme: (a) chymotrypsin; (b) trypsin; (c)carboxypeptidase; (d) C6H5N=C=S.arrow_forward
- Explain what is meant by:(i) a peptide linkage(ii) a glycosidic linkage.arrow_forwardPhospholipids undergo saponification much like triglycerides. Draw the structure of a phospholipid meeting the followingcriteria. Then draw the products that would result from its saponification.(a) a cephalin containing stearic acid and oleic acidarrow_forwardAn octapeptide contains the following amino acids: Arg, Glu, His, Ile, Leu, Phe, Tyr, and Val. Carboxypeptidase treatment of the octapeptide forms Phe and a heptapeptide. Treatment of the octapeptide with chymotrypsin forms two tetrapeptides, A and B. Treatment of A with trypsin yields two dipeptides, C and D. Edman degradation cleaves the following amino acids from each peptide: Glu (octapeptide), Glu (A), Ile (B), Glu (C), and Val (D). Partial hydrolysis of tetrapeptide B forms Ile–Leu in addition to other products. Deduce the structure of the octapeptide and fragments A–D.arrow_forward
- Which Compound Is Oxidized To Benzoic Acid With K2Cr2O7 In Acidic Medium?arrow_forwardThere are 20 common, naturally occurring amino acids from which all proteins are derived (as will be discussed in Chapter 25), although many other less common amino acids have been isolated from natural sources. Valine is one of the 20 common amino acids, and it was used as a starting material in the laboratory synthesis of an uncommon amino acid (Tetrahedron 1997, 53, 1151-1156). During one of the steps in the synthesis, compound 1 was treated with HBr under conditions that favor radical addition, giving stereoisomers 2 and 3. Draw the structures of 2 and 3, and describe their stereoisomeric relationship. H. LOCH3 HBr 2 + 3 hv H (S)-valine Modify the given copies of compound 1 to drawthe structures of 2 and 3. You can use the single bond tool to add/remove double bonds. CH2 CH2 H,C- H3C- -CH, -CH, Edit Drawing Describe the relationship of 2 and 3. O They are enantiomers. O They are superimposable. They are diastereomers. O They are constitutional isomers.arrow_forward27. Which of the following statements about cholesterol is not correct? CH3 CH3 H. d но Cholesterol (a) Cholesterol is a steroid that contains a tetracyclic ring system. (b) Cholesterol is a steroid that contains 8 chiral carbons and can form 28 or 256 stereoisomers. (c) Each atom or group attached to a ring-junction carbon (i.e., carbons a -e) is in a trans or axial position. Because of this the tetracyclic ring system is mostly flat. (d) Cholesterol is used to synthesized vitamin D, bile acids, sex hormones, and adrenocorticoid hormones. (e) Cholesterol is not found in the cell membranes of animals.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



