
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 24, Problem 6PP
Interpretation Introduction
Interpretation:
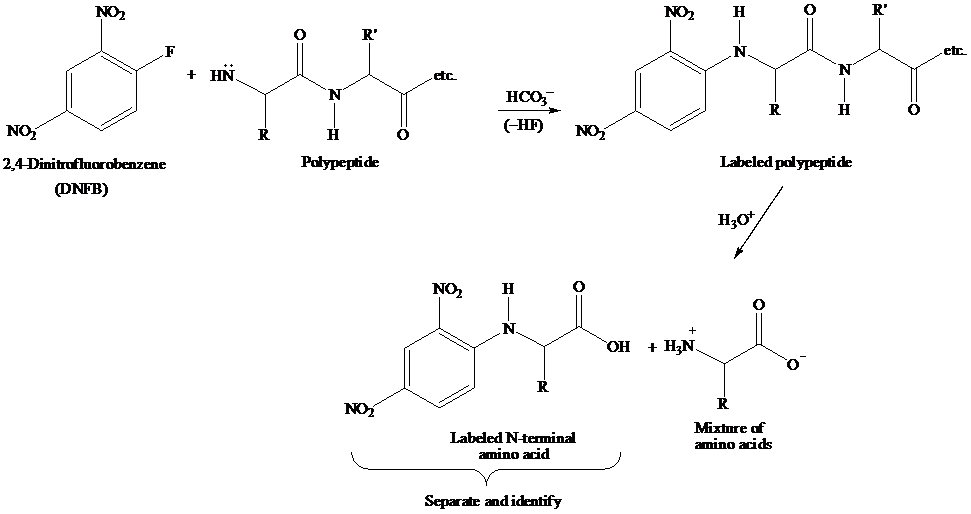
The reaction showing 2,4-dinitrofluorobenzene which could be used to identify the n-terminal amino acid of VAG is to be written. Further, the product of hydrolysis, when VKG is treated with 2,4-dinitrofluorobenzene, is to be identified.
Concept introduction:
The Sanger N-Terminal analysis is a method for sequence analysis of amino acid using 2,4-dinitrofluorobenzene (DNFB). The nucleophilic
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Glutathione (GSH) is a tripeptide that serves as a mild reducing agent to detoxify peroxides and maintain the cysteineresidues of hemoglobin and other red blood cell proteins in the reduced state. Complete hydrolysis of glutathione givesGly, Glu, and Cys. Treatment of glutathione with carboxypeptidase gives glycine as the first free amino acid released.Treatment of glutathione with 2,4-dinitrofluorobenzene (Sanger reagent, Problem 24-21, page 1246), followed bycomplete hydrolysis, gives the 2,4-dinitrophenyl derivative of glutamic acid. Treatment of glutathione with phenylisothiocyanate does not give a recognizable phenylthiohydantoin, however.(a) Propose a structure for glutathione consistent with this information. Why would glutathione fail to give a normalproduct from Edman degradation, even though it gives a normal product from the Sanger reagent followed byhydrolysis?(b) Oxidation of glutathione forms glutathione disulfide (GSSG). Propose a structure for glutathione disulfide, and…
Draw three-dimensional representations of the following amino acids. Explain their structures.
(a) L-phenylalanine
(b) L-histidine
(c) D-serine
(d) L-tryptophan
Assign an R or S configuration to the chiral center in each amino acid.
(a) l-Phenylalanine (b) l-Glutamic acid (c) l-Methionine
Chapter 24 Solutions
Organic Chemistry
Ch. 24 - Prob. 1PPCh. 24 - Practice Problem 24.2 The guanidino group NHNHCNH2...Ch. 24 - Prob. 3PPCh. 24 - Prob. 4PPCh. 24 - Prob. 5PPCh. 24 - Prob. 6PPCh. 24 - Prob. 7PPCh. 24 - Practice Problem 24.8
Glutathione is a tripeptide...Ch. 24 - Prob. 9PPCh. 24 - Prob. 10PP
Ch. 24 - Prob. 11PPCh. 24 - Practice Problem 24.12 Show all steps in the...Ch. 24 - Practice Problem 24.13 The synthesis of a...Ch. 24 - Practice Problem 24.14
The terminal carboxyl...Ch. 24 - Prob. 15PPCh. 24 - Prob. 16PPCh. 24 - (a) Which amino acids in Table 24.1 have more than...Ch. 24 - Prob. 18PCh. 24 - 24.19 (a) On the basis of the following sequence...Ch. 24 - Prob. 20PCh. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - The enzyme lysozyme and its mechanism are...Ch. 24 - Prob. 2LGP
Knowledge Booster
Similar questions
- Show how the following amino acids might be formed in the laboratory by reductiveamination of the appropriate a@ketoacid.(a) alanine (b) leucine (c) serine (d) glutaminearrow_forwardShow how the following amino acids might be formed in the laboratory by reductiveamination of the appropriate a@ketoacid.(a) alaninearrow_forwardSomatostatin is a tetradecapeptide of the hypothalamus that inhibits the release of pituitary growth hormone. Its amino acid sequence has been determined by a combination of Edman degradations and enzymic hydrolysis experiments. On the basis of the following data, deduce the primary structure of somatostatin: 1. Edman degradation gave PTH-Ala. 2. Selective hydrolysis gave peptides having the following indicated sequences: Phe-Trp Thr-Ser-Cys Lys-Thr-Phe Thr-Phe-Thr-Ser-Cys Asn-Phe-Phe-Trp-Lys Ala-Gly-Cys-Lys-Asn-Phe 3. Somatostatin has a disulfide bridge.arrow_forward
- Tetrapeptide A is completely hydrolysed under acidic conditions to yield alanine, aspartic acid, isoleucine and valine. On neutralisation of the hydrolysate, an odour of ammonia is detected. The reaction of the tetrapeptide with phenylisothiocyanate, followed by mild hydrolysis yields no phenylthiohydantoin derivative. Incubation of A with carboxypeptidase A has no effect either. Explain...arrow_forwardShow how the Gabriel malonic ester synthesis could be used to prepare: (a) valine (b) phenylalanine (c) glutamic acid (d) leucinearrow_forwardFor each amino acid listed, tell whether its influence on tertiary structure is largely through hydrophobic interactions,hydrogen bonding, formation of salt bridges, covalent bonding, or some combination of these effects.(a) Tyrosine (b) Cysteine(c) Asparagine (d) Lysine(e) Tryptophan (f) Alanine(g) Leucine (h) Methioninearrow_forward
- Draw three-dimensional representations of the following amino acids.(a) l-phenylalanine (b) l-histidine (c) d-serine (d) l-tryptophanDraw three-dimensional representations of the following amino acids.(a) l-phenylalanine (b) l-histidine (c) d-serine (d) l-tryptophanarrow_forwardBradykinin is a linear nonapeptide released by blood plasma globulin in response to a wasp sting. It is a very potent pain-causing agent. Its constituent amino acids are 2R, G, 2F, 3P, S. The sue of 2,4-dinitrofluorobenzene and carboxypeptidase shows that both terminal residues are arginine. Partial hydrolysis of bradykinin gives the following di- and tripeptides: FS + PGF +PP + SPF + FR + RParrow_forward(a) The isoelectric point (pI) of phenylalanine is pH 5.5. Draw the structure of the major form of phenylalanine at pHvalues of 1, 5.5, and 11.(b) The isoelectric point of histidine is pH 7.6. Draw the structures of the major forms of histidine at pH values of 1, 4,7.6, and 11. Explain why the nitrogen in the histidine ring is a weaker base than the a-amino group.(c) The isoelectric point of glutamic acid is pH 3.2. Draw the structures of the major forms of glutamic acid at pH valuesof 1, 3.2, 7, and 11. Explain why the side-chain carboxylic acid is a weaker acid than the acid group next to thea-carbon atomarrow_forward
- Although tryptophan contains a heterocyclic amine, it is considered a neutral aminoacid. Explain why the indole nitrogen of tryptophan is more weakly basic than one ofthe imidazole nitrogens of histidine.arrow_forwardGive the products of periodic acid oxidation of each of the following. How many moles of reagent will be consumed per mole of substrate in each case? (a) d-Arabinose (b) d-Ribose (c) Methyl β-d-glucopyranosidearrow_forwardWrite a structural formula for the product formed by treatment of the N-terminal amino group with Sanger’s reagent and propose a mechanism for its formation.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
