
Interpretation:
When the starting
Concept Introduction:
Heck reaction:
A palladium-catalyzed reaction of the carbon group of a haloalkene substituted for a hydrogen on double bonded carbon of an alkene is said to be Heck reaction.
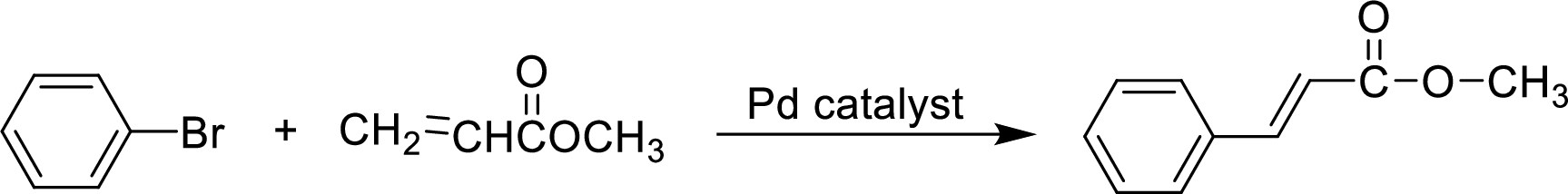
Explanation of Solution
The given reaction is
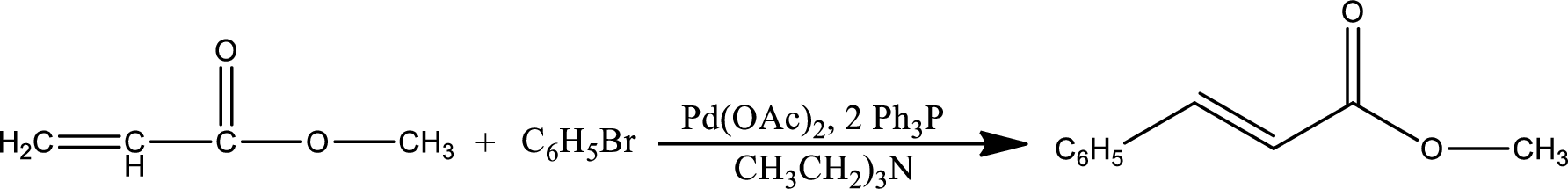
Mechanism:
Step-1:
Formation of Heck catalyst:
Heck catalyst s formed by the reduction of palladium from
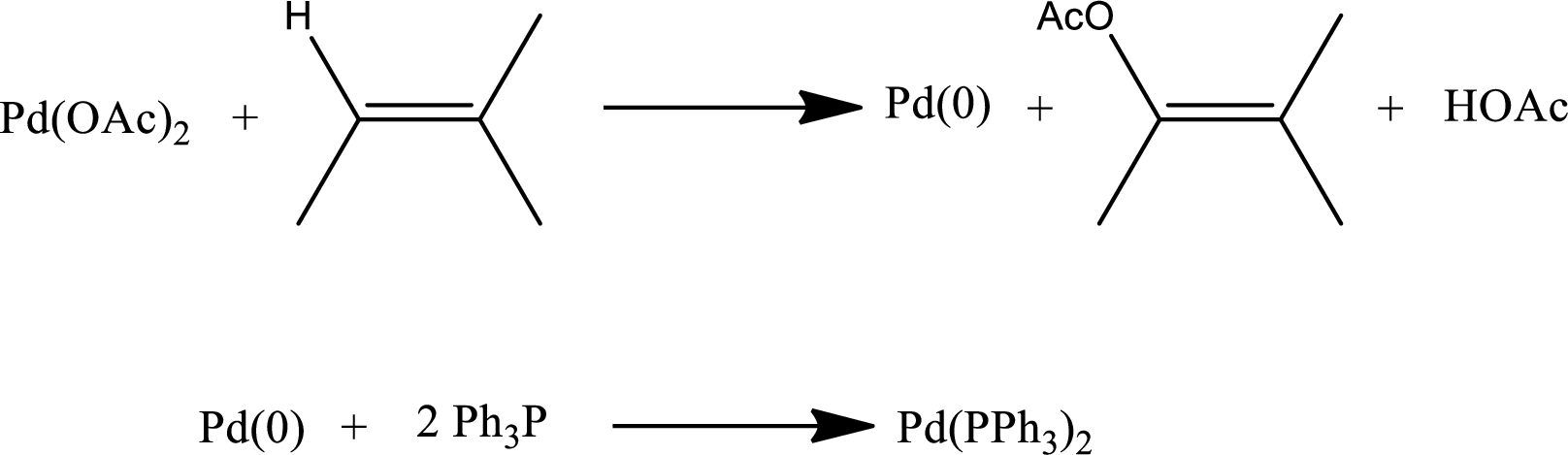
Step-2:
Catalytic cycle for the formation of product:
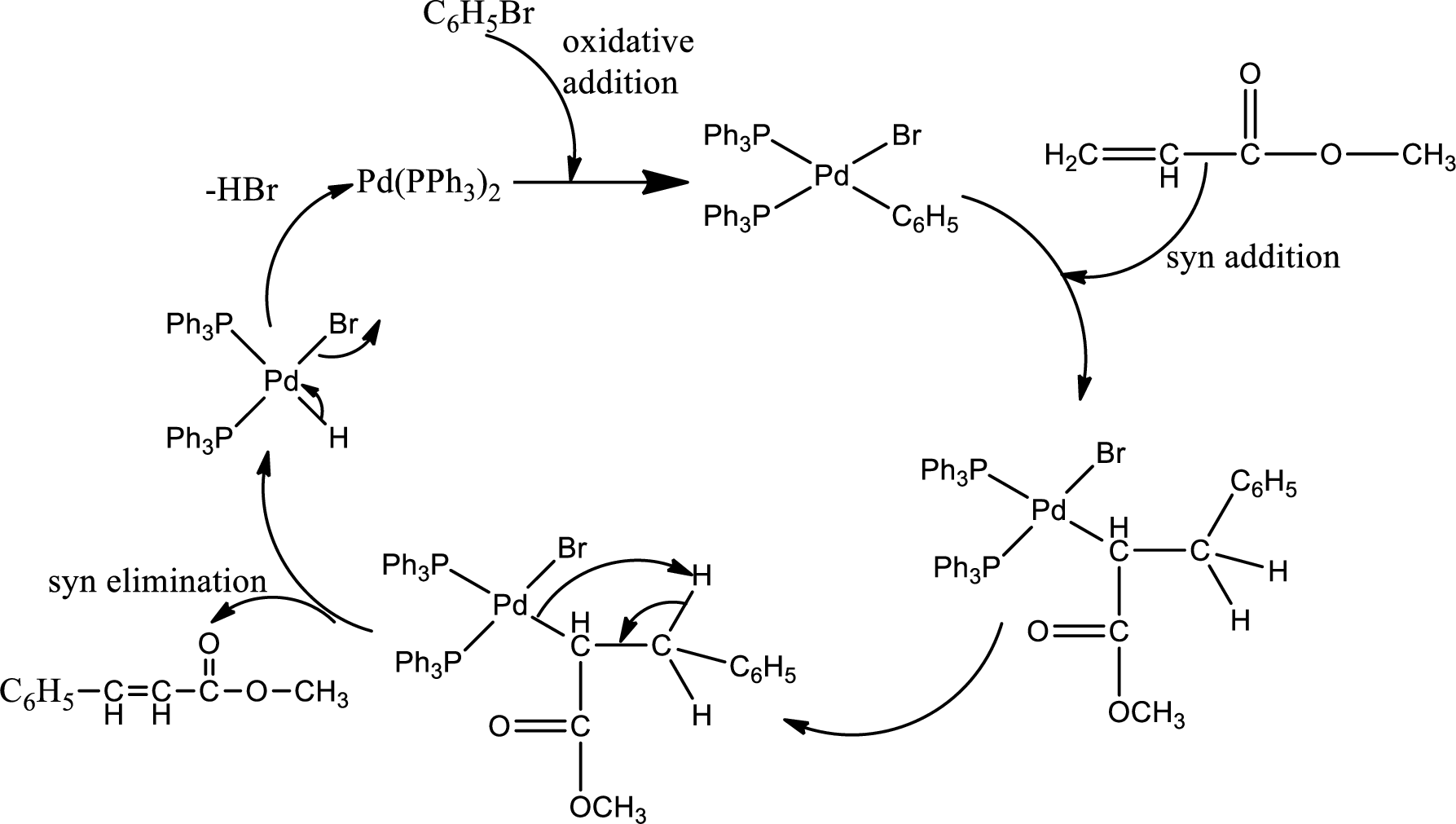
The addition of alkene to the catalyst is syn addition and elimination of product is also syn elimination. The inversion of configuration occurs in the addition and forms E-product.
Want to see more full solutions like this?
Chapter 24 Solutions
EP ORGANIC CHEMISTRY-OWL V2 ACCESS
- (b) Consider the reaction of 1-bromobutane with a large excess of ammonia (NH3). Draw the reactants, the transition state, andthe products. Note that the initial product is the salt of an amine (RNH3+ Br - ), which is deprotonated by the excess ammonia to give the amine.arrow_forwardDetermine whether the addition of a nucleophile to the O atom of a C=0 group is allowed or forbidden. If you determine that it is allowed, would you expect that elementary step to take place? Why or why not?arrow_forwardWhat elimination side reaction can occur from the following reaction in the presence of sulfuric acid? HINT: The product is an alkene but not 1-butene and the reaction has more than one step.arrow_forward
- Give 3 examples of a reaction mechanism of E1 that follows Zaitsev's rule.arrow_forwardCan you answer this question regarding this procedure: Discuss the Sn1 mechanism and then summarize how the different conditions impacted the rate. Look at leaving group, solvent, and substitution. What trends did you observe? Did any of the results turn out differently than you had expected? Do you think this is because of the mechanism or because of practical issues? Suggest possible ways to improve on the rate study for future trials.arrow_forward(a) In the lab, you are required to synthesize the target molecule 1 from the starting material provided in the following scheme. Upon completion of the reaction however, the compound you obtained was product 2, by reacting the starting material with water in the presence of H2SO4: OH OH Product 2 Starting Material Target Molecule 1 Based on your observations and knowledge of chemistry, answer the following questions: (i) Outline a detailed reaction mechanism to clearly explain the formation of product 2. (ii) Justify why product 2 was formed. 2.arrow_forward
- Alkynes do not react directly with aqueous acid as do alkenes, but will do so in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the OH group adds to the more highly substituted carbon and the H adds to the less highly substituted carbon. The initial product of the reaction is a vinyl alcohol, also called an enol. The enol immediately rearranges to a more stable ketone via tautomerization. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions -X티 Hö: H-O -CH3 -CH3 H30*arrow_forwardThe rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forward(d) Consider the following catalyzed and non-catalyzed pathways of a substitution reaction of bromoethane. Draw an energy diagram for each of the following reactions (label all axes and include reactants, intermediates (if any) and products). CH3CH₂Br + HO CH³CH₂OH + B (non-catalyzed) ное CH3CH₂Br + CH3CH₂OH + 1 (catalyzed) CH3CH₂ + Brarrow_forward
- Does the reaction likely proceed by the Su1, Su2, E1, or E2 mechanism?arrow_forwardWrite whether it will be E1, E2, SN1, and/or SN2 Show the mechanism(s) Write the MAJOR product(s) State the stereochemistry of the product(s), when applicable please do them in the form providedarrow_forwardWhen the Pd(0)-catalyzed reactions covered in this chapter are run with a slight pres- sure of carbon monoxide, a ketone is often created as the product. For example, the following Stille coupling conditions with added CO give the product shown. Write a mechanism for how this reaction could occur using the organometallic mechanistic steps introduced in this chapter, along with new steps that would be required in this transformation. Hint: CO can coordinate to Pd and insert into Pd-C bonds. n-BugSn Pd(PPH3)4, COarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

