
Organic Chemistry
11th Edition
ISBN: 9781118133576
Author: T. W. Graham Solomons, Craig Fryhle
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 24, Problem 21P
Interpretation Introduction
Interpretation:
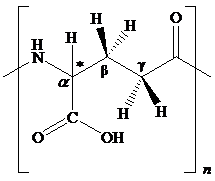
The structural feature of polyglutamic acid and the chemical change for given transformation are to be explained.
Concept introduction:
The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The peptide Proline-Serine-Alanine-Phenylalanine-Glutamine is present at pH 7. Draw the peptide and include stereochemistry.
Serine esterase contains a catalytic triad at its active site. Which amino acid in serine esterase is responsible for mediating general acid catalysis for the breakdown of tetrahedral intermediate to the carboxyl product?
Explain.
The amino acid sequence shown below is a short excerpt (residues 130-172) from the rod domain of an
a-keratin-like protein. Predict the effect of each of the amino acid substitutions (stabilize, destabilize, no effect) and
explain your reasoning.
130 - ATEFKTRFDDAALRANDIQL VIRSNEITADLIKDRLDQITTYY - 172
a) Substitution of S in place of A at position 140.
b) Substitution of V in place of I at position 147.
Chapter 24 Solutions
Organic Chemistry
Ch. 24 - Prob. 1PPCh. 24 - Practice Problem 24.2 The guanidino group NHNHCNH2...Ch. 24 - Prob. 3PPCh. 24 - Prob. 4PPCh. 24 - Prob. 5PPCh. 24 - Prob. 6PPCh. 24 - Prob. 7PPCh. 24 - Practice Problem 24.8
Glutathione is a tripeptide...Ch. 24 - Prob. 9PPCh. 24 - Prob. 10PP
Ch. 24 - Prob. 11PPCh. 24 - Practice Problem 24.12 Show all steps in the...Ch. 24 - Practice Problem 24.13 The synthesis of a...Ch. 24 - Practice Problem 24.14
The terminal carboxyl...Ch. 24 - Prob. 15PPCh. 24 - Prob. 16PPCh. 24 - (a) Which amino acids in Table 24.1 have more than...Ch. 24 - Prob. 18PCh. 24 - 24.19 (a) On the basis of the following sequence...Ch. 24 - Prob. 20PCh. 24 - Prob. 21PCh. 24 - Prob. 22PCh. 24 - Prob. 23PCh. 24 - Prob. 24PCh. 24 - The enzyme lysozyme and its mechanism are...Ch. 24 - Prob. 2LGPCh. 24 - 24.1 Write the structural formula of the principal...Ch. 24 - Prob. 2Q
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Outline the steps needed to synthesize the tetrapeptide Ala–Leu–Ile–Gly using the Merrifield technique.arrow_forwardGive the sequence of the following tetrapeptide:arrow_forwardTaking the structure of the amino acids into account, give a comparative discussion of these pK values : Alanine : 2.34 : 6.1 : 9.70 Histidine: 1.82 : 9.17arrow_forward
- Amino Acids and Proteins 1. What are the structural features of amino acids with an emphasis on essential amino acids? 2. Write the properties of amino acids: zwitterion, pka, pKb, amphoteric character, isoelectric point, and electrophoresis. 3. Write the mechanism of peptide bond formation and what are its structural features. 4. What are the different types of proteins and their function? 5. Explain the meaning and importance of the primary, secondary, tertiary, and quaternary structures of a protein and the factors that cause its denaturation.arrow_forwardA normal polypeptide and a mutant of the polypeptide were hydrolyzed by an endopeptidase under the same conditions. The normal and mutant poly peptide differ by one amino acid. The fingerprints of the peptides obtained from the two polypeptides are shown below. What kind of amino acid substitution occurred as a result of the mutation? (That is, is the substituted amino acid more or less polar than the original amino acid? Is its pI lower or higher?)arrow_forwardThe first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an a-keto acid, a reaction catalyzed by an enzyme called a transaminase. The a-keto acid acceptor is often a-ketoglutarate. Modify the structures in the product to show the products of the transamination of cysteine. Be sure to show functional groups with the charge and number of attached hydrogen atoms appropriate for pH 7.4. transaminase + O=C H₂N-CH + CH₂ CH₂ CH₂ SH Incorrect H₂N || CH | CH₂ | CH₂ I || O || n | CH₂ T SHarrow_forward
- Draw the structure of the tetrapeptide Asp-Arg-Val-Tyr. Please show the appropriate stereochemistry of the natural amino acids in the resulting peptide. Please draw all ionizable groups in their neutral form.arrow_forwardA tripeptide on hydrolysis produced glycine, alanine and leucine. The structures of these amino acids are shown below. On reaction with Edman’s reagent, leucine was released as the phenylhydantoin. Treatment of the tripeptide with carboxypeptidase gave glycine. Draw the structure of the tripeptide.arrow_forwardAlthough the bond energy for the hydrogen bond in a vacuum is estimated to be about 20 kJ/mol, we find that each hydrogen bond in a folded protein contributes much less--probably less that 5 kJ/mol--to the enthalpy of protein stabilization. Suggest an explanation for this difference. Explain briefly.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning