
Concept explainers
a)
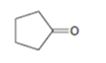
Interpretation:
The enone product expected from the aldol condensation of cyclopentanone is to be given.
Concept introduction:
To give:
The enone product expected from the aldol condensation of cyclopentanone.
b)
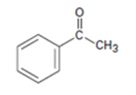
Interpretation:
The enone product expected from the aldol condensation of acetophenone is to be given.
Concept introduction:
Aldehydes and ketones with α-hydrogen undergo a base catalyzed carbonyl condensation reaction in aldol condensation. In this reaction two molecules of the reactant combine by forming a bond between α-carbon of one molecule and the carbonyl carbon of the second molecule. The product obtained, a β-hydroxyaldehyde or ketone upon heating eliminate a molecule of water to yield the enones, α,β-unsaturated aldehydes or ketones.
To give:
The enone product expected from the aldol condensation of acetophenone.
c)
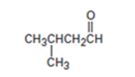
Interpretation:
The enone product expected from the aldol condensation of 2-methylbutanal is to be given.
Concept introduction:
Aldehydes and ketones with α-hydrogen undergo a base catalyzed carbonyl condensation reaction in aldol condensation. In this reaction two molecules of the reactant combine by forming a bond between α-carbon of one molecule and the carbonyl carbon of the second molecule. The product obtained, a β-hydroxyaldehyde or ketone upon heating eliminate a molecule of water to yield the enones, α,β-unsaturated aldehydes or ketones.
To give:
The enone product expected from the aldol condensation of 2-methylbutanal.
Trending nowThis is a popular solution!

Chapter 23 Solutions
Organic Chemistry
- Classify each of the following molecules as aromatic, antiaromatic, or nonaromatic. ZI NH Explanation Check O aromatic O antiaromatic O nonaromatic O aromatic O antiaromatic H O nonaromatic O aromatic O antiaromatic O nonaromatic ×arrow_forwardPart I. Draw the stepwise reaction mechanism of each product (a, b, c, d, e, f) HO HO OH НОН,С HO OH Sucrose HO CH₂OH H N N HO -H H -OH KMnO4, Heat H OH CH₂OH (d) Phenyl Osatriazole OH НОН,С HO HO + Glacial HOAC HO- HO CH₂OH OH HO Fructose (a) Glucose OH (b) H₂N HN (c) CuSO4-5H2O, ethanol H N N N HO ·H H OH H OH N CH₂OH OH (f) Phenyl Osazone H (e) Carboxy phenyl osatriazole Figure 2.1. Reaction Scheme for the Total Synthesis of Fine Chemicalsarrow_forwardWhich molecule is the most stable? Please explain.arrow_forward
- =Naming benzene derivatives Name these organic compounds: structure C1 CH3 name ☐ CH3 ப C1 × ☐arrow_forwardBlocking Group are use to put 2 large sterically repulsive group ortho. Show the correct sequence toconnect the reagent to product with the highest yield possible. * see image **NOTE: The compound on the left is the starting point, and the compound on the right is the final product. Please show the steps in between to get from start to final, please. These are not two different compounds that need to be worked.arrow_forwardI dont understand this.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

