
Concept explainers
To Compare: Ethane, ethene and ethyne.
Explanation of Solution
Introduction: Hydrocarbons having single bonds between atoms are classified as
Ethane,
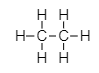
The bond between its two carbon atoms is a single covalent bond. Each carbon is attached to three hydrogen atoms by single bond.
Ethene,
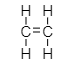
The bond between its two carbon atoms is double covalent bond. Each carbon is attached to two hydrogen atoms by single bond.
Ethyne,
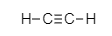
The bond between its two carbon atoms is triple covalent bond. Each carbon is attached to one hydrogen atoms by single bond.
Conclusion:
Ethane, ethene and ethyne each contains two carbon atoms, but has different type of carbon-carbon bond. Ethane has a single carbon-carbon bond, whereas ethene and ethyne have double and triple carbon-carbon bond respectively.
Chapter 23 Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Additional Science Textbook Solutions
College Physics: A Strategic Approach (3rd Edition)
Modern Physics
The Cosmic Perspective Fundamentals (2nd Edition)
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
Physics for Scientists and Engineers with Modern Physics
Lecture- Tutorials for Introductory Astronomy
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





