
Interpretation:
The molecular formula, molecular weight and
Concept introduction:
The compounds having similar chemical formula but different structures are known as isomers.
The compounds having similar chemical or molecular formula but different connectivity is known as constitutional isomers.
Molecular formula of a compound contains
Molecular weight is defined as the addition of
Functional group is defined as the group of atoms or bonds present in a compound which is responsible for the
Answer to Problem 63A
The molecular mass and molecular weight of both glucose and fructose is same.
Fructose contains
Explanation of Solution
Glucose consists of single unit of sugar which can’t be hydrolyzed into simpler molecule. Glucose is obtained by the photosynthesis process and also found in plants.
Fructose consists of single unit of sugar which can’t be hydrolyzed into simpler molecule.
Glucose and fructose are structural isomers of each other implies they have same molecular formula that is
Molecular formula of glucose and fructose =
Molecular weight of glucose and fructose
=
=
Now, the structure of glucose and fructose is:
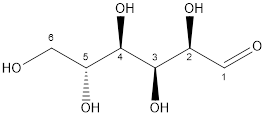
Glucose
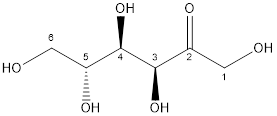
Fructose
Glucose and fructose both have six carbon atoms and five alcohol groups. But, fructose contains ketone (-C=O) functional group whereas glucose contains aldehyde (-CHO) functional group.
Chapter 23 Solutions
Glencoe Chemistry: Matter and Change, Student Edition
Additional Science Textbook Solutions
Campbell Essential Biology (7th Edition)
The Cosmic Perspective (8th Edition)
Cosmic Perspective Fundamentals
Microbiology: An Introduction
Human Anatomy & Physiology (2nd Edition)
Anatomy & Physiology (6th Edition)
- For the single step reaction: A + B → 2C + 25 kJ If the activation energy for this reaction is 35.8 kJ, sketch an energy vs. reaction coordinate diagram for this reaction. Be sure to label the following on your diagram: each of the axes, reactant compounds and product compounds, enthalpy of reaction, activation energy of the forward reaction with the correct value, activation energy of the backwards reaction with the correct value and the transition state. In the same sketch you drew, after the addition of a homogeneous catalyst, show how it would change the graph. Label any new line "catalyst" and label any new activation energy.arrow_forwardHow many grams of C are combined with 3.75 ✕ 1023 atoms of H in the compound C5H12?arrow_forwarde. f. CH3O. יון Br NaOCH3 OCH 3 Br H₂Oarrow_forward
- Don't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward5. A solution of sucrose is fermented in a vessel until the evolution of CO2 ceases. Then, the product solution is analyzed and found to contain, 45% ethanol; 5% acetic acid; and 15% glycerin by weight. If the original charge is 500 kg, evaluate; e. The ratio of sucrose to water in the original charge (wt/wt). f. Moles of CO2 evolved. g. Maximum possible amount of ethanol that could be formed. h. Conversion efficiency. i. Per cent excess of excess reactant. Reactions: Inversion reaction: C12H22O11 + H2O →2C6H12O6 Fermentation reaction: C6H12O6 →→2C2H5OH + 2CO2 Formation of acetic acid and glycerin: C6H12O6 + C2H5OH + H₂O→ CH3COOH + 2C3H8O3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





