
(a)
Interpretation:
The number of signals of
Concept introduction:
Nuclear magnetic resonance spectroscopy is applied for the identification of the structure of molecules. The energy in the radiofrequency region is suitable for NMR. Nuclear magnetic resonance results from the spin of the nucleus of an atom. The value of I is obtained using the
Any nucleus with both an even atomic number and the mass number has 0 nuclear spins. There are a total of
However, energy levels become non-degenerate in the presence of a magnetic field.
Deuterated chloroform
The total signal intensity of each set of proton is given by the height of each set of steps. The integration value defines the relative number of each kind of proton in the molecule.
In NMR spectrum, the intensity of signals is plotted against the magnetic field or frequency. Nuclei that are non-equivalent show only one peak in the NMR spectrum. However, protons absorb at different frequencies that are non-equivalent.
An increase in the electron density that surrounds the nucleus shields it from the applied field. This results in a net decrease in the field experienced by the nucleus. The value of the observed chemical shift of the signal therefore decreases, and, on a typical NMR spectrum, the signal moves to the right, which is called an upfield shift because, at a constant frequency, a slightly higher applied magnetic field is required for resonance to occur. De-shielding is the effect of a decline in the electron density around a nucleus which leads to shifting in the peaks of a chemical shift towards left in the NMR spectrum that results in an increase in delta values, hence downshift.
Explanation of Solution
The decoupled
Isopropyl acetate has 4 different types of carbon as here also 2 methyl groups in the isopropyl group that have equivalent chemical environment. So they show 4 signals.
(b)
Interpretation:
The number of signals of
Concept introduction:
Nuclear magnetic resonance spectroscopy is applied for the identification of the structure of molecules. The energy in the radiofrequency region is suitable for NMR. Nuclear magnetic resonance results from the spin of the nucleus of an atom. The value of I is obtained using the atomic number and the mass number of an atom. The non-zero magnetic moment of an isotopic nucleus is detectable by the NMR technique.
Any nucleus with both an even atomic number and the mass number has 0 nuclear spins. There are a total of
However, energy levels become non-degenerate in the presence of a magnetic field.
Deuterated chloroform
The total signal intensity of each set of proton is given by the height of each set of steps. The integration value defines the relative number of each kind of proton in the molecule.
In NMR spectrum, the intensity of signals is plotted against the magnetic field or frequency. Nuclei that are non-equivalent show only one peak in the NMR spectrum. However, protons absorb at different frequencies that are non-equivalent.
An increase in the electron density that surrounds the nucleus shields it from the applied field. This results in a net decrease in the field experienced by the nucleus. The value of the observed chemical shift of the signal therefore decreases, and, on a typical NMR spectrum, the signal moves to the right, which is called an upfield shift because, at a constant frequency, a slightly higher applied magnetic field is required for resonance to occur. De-shielding is the effect of a decline in the electron density around a nucleus which leads to shifting in the peaks of a chemical shift towards left in the NMR spectrum that results in an increase in delta values, hence downshift.
Explanation of Solution
The decoupled
(c)
Interpretation:
The number of signals for cyclohexane,
Concept introduction:
Nuclear magnetic resonance spectroscopy is applied for the identification of the structure of molecules. The energy in the radiofrequency region is suitable for NMR. Nuclear magnetic resonance results from the spin of the nucleus of an atom. The value of I is obtained using the atomic number and the mass number of an atom. The non-zero magnetic moment of an isotopic nucleus is detectable by the NMR technique.
Any nucleus with both an even atomic number and the mass number has 0 nuclear spins. There are a total of
However, energy levels become non-degenerate in the presence of a magnetic field.
Deuterated chloroform
The total signal intensity of each set of proton is given by the height of each set of steps. The integration value defines the relative number of each kind of proton in the molecule.
In NMR spectrum, the intensity of signals is plotted against the magnetic field or frequency. Nuclei that are non-equivalent show only one peak in the NMR spectrum. However, protons absorb at different frequencies that are non-equivalent.
An increase in the electron density that surrounds the nucleus shields it from the applied field. This results in a net decrease in the field experienced by the nucleus. The value of the observed chemical shift of the signal therefore decreases, and, on a typical NMR spectrum, the signal moves to the right, which is called an upfield shift because, at a constant frequency, a slightly higher applied magnetic field is required for resonance to occur. De-shielding is the effect of a decline in the electron density around a nucleus which leads to shifting in the peaks of a chemical shift towards left in the NMR spectrum that results in an increase in delta values, hence downshift.
Explanation of Solution
The decoupled
Cyclohexane has 1 type of carbon atom as it is symmetrical molecule with 6 planes of symmetry in it so 1 signal is obtained for it.
(d)
Interpretation:
The number of signals in the following structures in decoupled
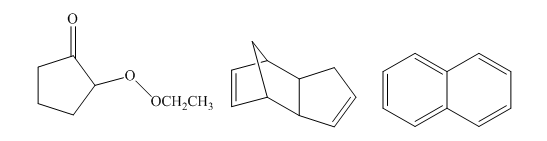
Concept introduction:
Nuclear magnetic resonance spectroscopy is applied for the identification of the structure of molecules. The energy in the radiofrequency region is suitable for NMR. Nuclear magnetic resonance results from the spin of the nucleus of an atom. The value of I is obtained using the atomic number and the mass number of an atom. The non-zero magnetic moment of an isotopic nucleus is detectable by the NMR technique.
Any nucleus with both an even atomic number and the mass number has 0 nuclear spins. There are a total of
However, energy levels become non-degenerate in the presence of a magnetic field.
Deuterated chloroform
The total signal intensity of each set of proton is given by the height of each set of steps. The integration value defines the relative number of each kind of proton in the molecule.
In NMR spectrum, the intensity of signals is plotted against the magnetic field or frequency. Nuclei that are non-equivalent show only one peak in the NMR spectrum. However, protons absorb at different frequencies that are non-equivalent.
An increase in the electron density that surrounds the nucleus shields it from the applied field. This results in a net decrease in the field experienced by the nucleus. The value of the observed chemical shift of the signal therefore decreases, and, on a typical NMR spectrum, the signal moves to the right, which is called an upfield shift because, at a constant frequency, a slightly higher applied magnetic field is required for resonance to occur. De-shielding is the effect of a decline in the electron density around a nucleus which leads to shifting in the peaks of a chemical shift towards left in the NMR spectrum that results in an increase in delta values, hence downshift.
Explanation of Solution
The decoupled
The number of signals in the structures is indicated as follows:
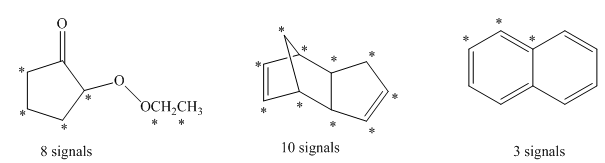
Want to see more full solutions like this?
Chapter 23 Solutions
Laboratory Techniques in Organic Chemistry
- Describe the 1H-NMR spectrum of 4-bromoaniline (chemical shift, integration, multiple). Make a table and describe the results.arrow_forwardHow many signals in 1H NMR spectrum of methyl 3-nitrobenzoate? Identify their chemical shifts and the multiplicity.arrow_forward6. The following compound is a carboxylic acid that contains a bromine atom: C,H,02Br. The peak at 10.97 ppm was moved onto the chart (which runs only from 0-8 ppm) for clarity. What is the structure of the compound? 400 300 200 100 CPS H NMR 60 MHz Integral = 3 CaH;O>Br Integral = 1 Integral = 1 Integral = 2 8.0 7.0 6.0 5.0 3.0 2.0 1.0 O PPMarrow_forward
- How many signals would you expect to observe in the 1H -NMR spectrum of 1,3-dichloropropane? What would be the multiplicity (splitting) of each signal?arrow_forwardEach of the following molecules has a unique 1H NMR spectrum. Choose the molecule(s) that will only show two signals, with an integration ratio of 2:3, in their 1H NMR spectrum.arrow_forwardConsider the aromatic compound 4-isopropyl-benzonitrile. (Benzonitrile is a benzene ring with the nitrile group on position 1.) How many signals for non-equivalent types of protons will be in its proton NMR spectrum?arrow_forward
- Consider the 13C NMR of benzamide. How many carbon signals would you expect to find for this compound? O 1) 8 O 2) 6 O 3) 4 4) 7 O 5) 5 6) 9arrow_forwardHow many signals will be found in the 13c NMR of 2 propanol at room temperaturearrow_forwardThe line of integration of the two signals in the 1H-NMR spectrum of a ketone with the molecular formula C7H14O rises 62 and 10 chart divisions, respectively. Calculate the number of hydrogens giving rise to each signal and propose a structural formula for this ketone.arrow_forward
- In a proton NMR spectrum, indicate the number of peaks and their multiplicity for the following compounds:CH3 - CO - CH3CH3 - CH2 - CO - CH3Cl - CH = CH2ortho-chloromethylbenzenearrow_forwardThe 1H NMR spectra of two carboxylic acids with molecular formula C3H5O2Cl are shown below. Identify the carboxylic acids. (The “offset” notation means that the farthest-left signal has been moved to the right by the indicated amount to fit on the spectrum; thus, the signal at 9.8 ppm offset by 2.4 ppm has an actual chemical shift of 9.8 + 2.4 = 12.2 ppm.)arrow_forwardAnalyze the 13C-NMR spectrum of C8H9NO given below and draw the structure of the compound.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





