
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 67P
7 An enzyme has the following pH dependence:
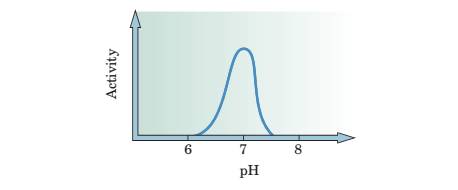
At what pH do you think this enzyme works best?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are false
(f) SO:
Best Lewis Structure
3
e group geometry:_
shape/molecular geometry:,
(g) CF2CF2
Best Lewis Structure
polarity:
e group arrangement:_
shape/molecular geometry:
(h) (NH4)2SO4
Best Lewis Structure
polarity:
e group arrangement:
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Sketch (with angles):
1.
Problem Set 3b
Chem 141
For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing
bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the
molecule is polar or non-polar (iv)
(a) SeF4
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
(b) AsOBr3
Best Lewis Structure
e group arrangement:_
shape/molecular geometry:
polarity:
Sketch (with angles):
Sketch (with angles):
Chapter 22 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 22.1 - Prob. 22.1QCCh. 22.2 - Prob. 22.2QCCh. 22.3 - Prob. 22.3QCCh. 22.4 - Which of the following is true regarding enzymes?...Ch. 22.5 - Match the description of the enzyme process with...Ch. 22 - What is the difference between a catalyst and an...Ch. 22 - What are ribozymes made of?Ch. 22 - Would a lipase hydrolyze two triglycerides, one...Ch. 22 - Compare the activation energy in uncatalyzed...Ch. 22 - Prob. 5P
Ch. 22 - Prob. 6PCh. 22 - Prob. 7PCh. 22 - Monoamine oxidases are important enzymes in brain...Ch. 22 - Prob. 9PCh. 22 - 0 What kind of reaction does each of the following...Ch. 22 - Prob. 11PCh. 22 - In most enzyme-catalyzed reactions, the rate of...Ch. 22 - 5 At a very low concentration of a certain...Ch. 22 - 6 If we wish to double the rate of an...Ch. 22 - 7 A bacterial enzyme has the following...Ch. 22 - 8 The optimal temperature for the action of...Ch. 22 - 9 The activity of pepsin was measured at various...Ch. 22 - Prob. 18PCh. 22 - Prob. 19PCh. 22 - Prob. 20PCh. 22 - Prob. 21PCh. 22 - 4 What kind of chemical reaction occurs most...Ch. 22 - 5 Which of the following is a correct statement...Ch. 22 - Prob. 24PCh. 22 - 7 Enzymes are long protein chains, usually...Ch. 22 - Prob. 26PCh. 22 - Prob. 27PCh. 22 - 0 Can the product of a reaction that is part of a...Ch. 22 - 1 What is the difference between a zymogen and a...Ch. 22 - 2 The enzyme trypsin is synthesized by the body in...Ch. 22 - Prob. 31PCh. 22 - Prob. 32PCh. 22 - Prob. 33PCh. 22 - Prob. 34PCh. 22 - Prob. 35PCh. 22 - Prob. 36PCh. 22 - Prob. 37PCh. 22 - Prob. 38PCh. 22 - 1 After a heart attack, the levels of certain...Ch. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - 5 Chemists who have been exposed for years to or...Ch. 22 - 6 Which enzyme preparation is given to patients...Ch. 22 - 7 Chymotrypsm is secreted by the pancreas and...Ch. 22 - Prob. 46PCh. 22 - Prob. 47PCh. 22 - Prob. 48PCh. 22 - Prob. 49PCh. 22 - Prob. 50PCh. 22 - Prob. 51PCh. 22 - Prob. 52PCh. 22 - Prob. 53PCh. 22 - Prob. 54PCh. 22 - Prob. 55PCh. 22 - Prob. 56PCh. 22 - Prob. 57PCh. 22 - Prob. 58PCh. 22 - Prob. 59PCh. 22 - Prob. 60PCh. 22 - 1 Food can be preserved by inactivation of enzymes...Ch. 22 - Prob. 62PCh. 22 - 3 Would you expect to find active digestive...Ch. 22 - Prob. 64PCh. 22 - Prob. 65PCh. 22 - Prob. 66PCh. 22 - 7 An enzyme has the following pH dependence: At...Ch. 22 - Prob. 68PCh. 22 - Prob. 69PCh. 22 - 0 Nerve gases operate by forming covalent bonds at...Ch. 22 - 1 What would be the appropriate name for an enzyme...Ch. 22 - Prob. 72PCh. 22 - 3 A liver enzyme is made of four subunits: 2A and...Ch. 22 - Prob. 74PCh. 22 - Prob. 75PCh. 22 - Prob. 76PCh. 22 - Prob. 77PCh. 22 - Prob. 78PCh. 22 - Prob. 79PCh. 22 - Prob. 80PCh. 22 - Prob. 81P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License