
Bundle: Chemistry, Loose-Leaf Version, 10th + OWLv2, 4 terms (24 months) Printed Access Card
10th Edition
ISBN: 9781337537933
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 33E
Name each of the following.
a. 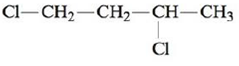
b. CH3CH2CH2CCl3
c. 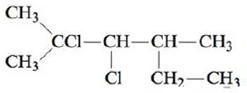
d. CH2FCH2F
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The structure of the fatty acid, palmitoleic acid, is shown below. How would you classify this fatty acid?
a. polyunsaturated
b. waxy
c. monounsaturated
d. saturated
Name HO-CH2CH2CH2CH3
A. 1-butanol
B. propanol
C.Butane
D.ethanol
3 amino acids joined together by two peptide bonds.a. Dipeptideb. Tripeptidec. Tetrapeptided. Polypeptide
An amino acid that can form disulfide bondsa. Cysteineb. Methioninec. Cystined. Serine
Chapter 22 Solutions
Bundle: Chemistry, Loose-Leaf Version, 10th + OWLv2, 4 terms (24 months) Printed Access Card
Ch. 22 - What is a hydrocarbon? What is the difference...Ch. 22 - Prob. 2RQCh. 22 - What are aromatic hydrocarbons? Benzene exhibits...Ch. 22 - Summarize the nomenclature rules for alkanes,...Ch. 22 - What functional group distinguishes each of the...Ch. 22 - Distinguish between isomerism and resonance....Ch. 22 - Prob. 7RQCh. 22 - Prob. 8RQCh. 22 - Prob. 9RQCh. 22 - Prob. 10RQ
Ch. 22 - Prob. 11RQCh. 22 - Describe the structural differences between DNA...Ch. 22 - Prob. 1QCh. 22 - Prob. 2QCh. 22 - Prob. 3QCh. 22 - Prob. 4QCh. 22 - Prob. 5QCh. 22 - Prob. 6QCh. 22 - Prob. 7QCh. 22 - Give an example reaction that would yield the...Ch. 22 - Prob. 9QCh. 22 - Prob. 10QCh. 22 - Prob. 11QCh. 22 - Prob. 12QCh. 22 - Is the primary, secondary, or tertiary structure...Ch. 22 - Prob. 14QCh. 22 - Prob. 15ECh. 22 - Prob. 16ECh. 22 - Draw all the structural isomers for C8H18 that...Ch. 22 - Draw all the structural isomers for C8H18 that...Ch. 22 - Prob. 19ECh. 22 - Prob. 20ECh. 22 - Draw the structural formula for each of the...Ch. 22 - Prob. 22ECh. 22 - Prob. 23ECh. 22 - Name each of the following cyclic alkanes, and...Ch. 22 - Prob. 25ECh. 22 - Draw the structures for two examples of...Ch. 22 - Name each of the following alkenes. a. CH2 = CH ...Ch. 22 - Name each of the following alkenes or alkynes. a....Ch. 22 - Prob. 29ECh. 22 - Give the structure for each of the following. a....Ch. 22 - Prob. 31ECh. 22 - Cumene is the starting material for the industrial...Ch. 22 - Name each of the following. a. b. CH3CH2CH2CCl3 c....Ch. 22 - Prob. 34ECh. 22 - There is only one compound that is named...Ch. 22 - Prob. 36ECh. 22 - Prob. 37ECh. 22 - Prob. 38ECh. 22 - Draw all the structural isomers of C5H10. Ignore...Ch. 22 - Prob. 40ECh. 22 - Draw all the structural and geometrical (cistrans)...Ch. 22 - Draw all the structural and geometrical (cistrans)...Ch. 22 - Draw all structural and geometrical (cistrans)...Ch. 22 - Prob. 44ECh. 22 - Prob. 45ECh. 22 - Prob. 46ECh. 22 - Prob. 47ECh. 22 - Prob. 48ECh. 22 - If one hydrogen in a hydrocarbon is replaced by a...Ch. 22 - There are three isomers of dichlorobenzene, one of...Ch. 22 - Identify each of the following compounds as a...Ch. 22 - Prob. 52ECh. 22 - Mimosine is a natural product found in large...Ch. 22 - Minoxidil (C9H15N5O) is a compound produced by...Ch. 22 - For each of the following alcohols, give the...Ch. 22 - Prob. 56ECh. 22 - Prob. 58ECh. 22 - Prob. 59ECh. 22 - Prob. 60ECh. 22 - Prob. 61ECh. 22 - Prob. 62ECh. 22 - Prob. 63ECh. 22 - Prob. 64ECh. 22 - Prob. 65ECh. 22 - Prob. 66ECh. 22 - Prob. 67ECh. 22 - Prob. 68ECh. 22 - Prob. 69ECh. 22 - Prob. 70ECh. 22 - Prob. 71ECh. 22 - Prob. 72ECh. 22 - Prob. 73ECh. 22 - Prob. 74ECh. 22 - Prob. 75ECh. 22 - Prob. 76ECh. 22 - Prob. 77ECh. 22 - Prob. 78ECh. 22 - Super glue contains methyl cyanoacrylate, which...Ch. 22 - Prob. 80ECh. 22 - Prob. 81ECh. 22 - Prob. 82ECh. 22 - Prob. 83ECh. 22 - Prob. 84ECh. 22 - Prob. 85ECh. 22 - Prob. 86ECh. 22 - Prob. 87ECh. 22 - Prob. 88ECh. 22 - Prob. 89ECh. 22 - Prob. 90ECh. 22 - Aspartame, the artificial sweetener marketed under...Ch. 22 - Prob. 92ECh. 22 - Prob. 93ECh. 22 - Draw the structures of the tripeptides glyalaser...Ch. 22 - Prob. 95ECh. 22 - Prob. 96ECh. 22 - Prob. 97ECh. 22 - In general terms, what does the tertiary structure...Ch. 22 - Give an example of amino acids that could give...Ch. 22 - What types of interactions can occur between the...Ch. 22 - Prob. 101ECh. 22 - Prob. 102ECh. 22 - Prob. 103ECh. 22 - Prob. 104ECh. 22 - Prob. 105ECh. 22 - Prob. 106ECh. 22 - Prob. 107ECh. 22 - Prob. 108ECh. 22 - Prob. 109ECh. 22 - Prob. 110ECh. 22 - Prob. 111ECh. 22 - Prob. 114ECh. 22 - Prob. 115ECh. 22 - Prob. 116ECh. 22 - Prob. 117ECh. 22 - Prob. 118ECh. 22 - The base sequences in mRNA that code for certain...Ch. 22 - Prob. 120ECh. 22 - Prob. 121AECh. 22 - Prob. 122AECh. 22 - Prob. 123AECh. 22 - Prob. 124AECh. 22 - Prob. 125AECh. 22 - Prob. 126AECh. 22 - Prob. 127AECh. 22 - Prob. 128AECh. 22 - Explain why methyl alcohol is soluble in water in...Ch. 22 - Prob. 130AECh. 22 - Prob. 131AECh. 22 - Prob. 132AECh. 22 - Prob. 133AECh. 22 - Prob. 134AECh. 22 - Prob. 135AECh. 22 - Prob. 136AECh. 22 - Prob. 137AECh. 22 - Prob. 138AECh. 22 - Prob. 139AECh. 22 - Prob. 140AECh. 22 - Prob. 141AECh. 22 - a. Use bond energies (see Table 3-3) to estimate H...Ch. 22 - Prob. 143AECh. 22 - Prob. 144AECh. 22 - All amino acids have at least two functional...Ch. 22 - Prob. 146AECh. 22 - Prob. 147AECh. 22 - In glycine, the carboxylic acid group has Ka = 4.3...Ch. 22 - Name each of the following alkanes. a....Ch. 22 - Prob. 150CWPCh. 22 - Name each of the following cyclic alkanes. a. b....Ch. 22 - Name each of the following alkenes and alkynes. a....Ch. 22 - a. Name each of the following alcohols. b. Name...Ch. 22 - Prob. 154CWPCh. 22 - Prob. 155CWPCh. 22 - Prob. 156CWPCh. 22 - Prob. 157CPCh. 22 - Estimate H for the following reactions using bond...Ch. 22 - Prob. 159CPCh. 22 - Prob. 160CPCh. 22 - Prob. 161CPCh. 22 - Consider a sample of a hydrocarbon at 0.959 atm...Ch. 22 - Prob. 163CPCh. 22 - Prob. 164CPCh. 22 - Prob. 165CPCh. 22 - Prob. 166CPCh. 22 - Prob. 167CPCh. 22 - Prob. 168CPCh. 22 - Prob. 169CPCh. 22 - Alcohols are very useful starting materials for...Ch. 22 - A chemical breathalyzer test works because ethanol...Ch. 22 - Estradiol is a female hormone with the following...Ch. 22 - Prob. 173IPCh. 22 - Prob. 174IPCh. 22 - Prob. 175MPCh. 22 - Prob. 176MP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For each of the following alcohols, give the systematic name and specify whether the alcohol is primary, secondary, or tertiary. a. b. c.arrow_forwardFatty acids are carboxylic acids that have long hydrocarbon chains attached to a carboxylate group. How does a saturated fatty acid differ from an unsaturated fatty acid? How are they similar?arrow_forwardWhich of the compounds can exist as enantiomers? A. Isopropyl chloride B. Bromochloromethane C. Sec-butyl chloride D. 1-chloro-2methylpentane a. A and B b. B and D c. C and D d. B and Carrow_forward
- what functional group ch2ch3 is?arrow_forwardIdentify the organic functional groups and reaction type for the following reaction.The reactant is a(n)a. ketopentoseb. aldopentosec. ketotriosed. alcohol pentosee. carboxylic acid tetroseThe product is a(n)a. aldohexosesb. alcohol pentosesc. carboxylic acid pentosesd. deoxypentosee. ketopentosesThe reaction type is:a. hemiacetal formationb. reduction (hydrogenation)c. hydrolysisd. acetal formatione. mutarotationf. oxidation (benedict's)arrow_forwardWhat functional groups are present in a carbohydrate molecule? a. Carboxyl and carbonyl groups b. Alcohol and carboxyl groups c. Hydroxyl and carbonyl groups d. Hydroxyl and hydrogen groupsarrow_forward
- Name the organic structure. H. CH2CH2CH2CH3 H.arrow_forwardWhat type of compound does the following structure belong to? Select all that apply. O -R CH,O O CHOR CH₂OR" A. Oil B. Glycerol C. Soap D. Fatarrow_forwardA. Identify and name the functional group in each of the following. 1. Снзсоснз 2. снзосн2сHз 3. CH3CH=CH2 CH3CH2COOH 5. CH3CH2CHO 6. CH3CH2CH20H 4.arrow_forward
- Name CH3CH2CH2CH2CH2Cl A. 1-chloropentane B. Butanol C. pentamine D. pentanochloroarrow_forwardThe group having the condensed structural formula “CH3CH2CH(CH3)–” is called ___ .arrow_forwardClassify the functional groups in the following steroid (choose all that apply).CHOOSE ALL THAT APPLY a. ester b. ketone c. tertiary alcohol d. primary alcohol e. amide f. alkyne g. aldehyde h. carboxylic acid i. alkene j. secondary alcoholarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div  Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY