
Concept explainers
Draw the product formed when phenylacetonitrile
a.
b.
c.
d.
e.
f.
(a)
Interpretation: The product formed from the treatment of phenylacetonitrile
Concept introduction: Hydrolysis of nitriles in acidic medium converts them to the corresponding carboxylic acids. The reaction involves two parts; that is nucleophilic addition followed by nucleophilic acyl substitution.
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
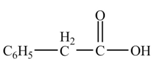
Explanation of Solution
The given compound is a nitrile.
Hydrolysis of nitriles in acidic medium converts them to the corresponding carboxylic acids. The reaction involves two parts; that is nucleophilic addition followed by nucleophilic acyl substitution. Phenylacetonitrile
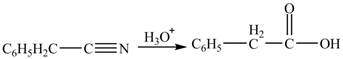
Figure 1
The product formed from the treatment of phenylacetonitrile
(b)
Interpretation: The product formed from the treatment of phenylacetonitrile
Concept introduction: Phenylacetonitrile undergo hydrolysis in basic medium to form corresponding carboxylate ion. The mechanism of the reaction involves two parts. The first part is conversion of nitrile to a
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
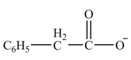
Explanation of Solution
The given compound is a nitrile.
Hydrolysis of nitriles in basic medium converts them to the corresponding carboxylate anion. Nitriles react with
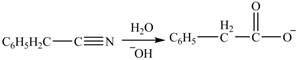
Figure 2
The product formed from the treatment of phenylacetonitrile
(c)
Interpretation: The product formed from the treatment of phenylacetonitrile
Concept introduction: The Grignard reagents are organometallic compounds having the general formula
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
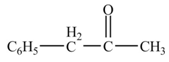
Explanation of Solution
The given compound is a nitrile.
Nitriles react with both Grignard reagent, and organolithium reagents, followed by hydrolysis to yield ketones. The reaction utilizes
Phenylacetonitrile undergoes Grignard reaction with
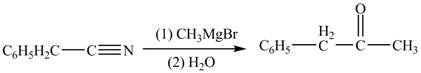
Figure 3
The product formed from the treatment of phenylacetonitrile
(d)
Interpretation: The product formed from the treatment of phenylacetonitrile
Concept introduction: Organolithium reagents are organometallic compounds having the general formula
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
Explanation of Solution
The given compound is a nitrile.
Nitriles react with both Grignard reagent, and organolithium reagents, followed by hydrolysis to yield ketones. The reaction utilizes
The treatment of phenylacetonitrile with
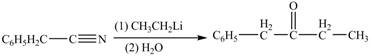
Figure 4
The product formed from the treatment of phenylacetonitrile
Interpretation: The product formed from the treatment of phenylacetonitrile
(e)
Concept introduction:
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
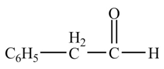
Explanation of Solution
Diisobutylaluminiumhydride
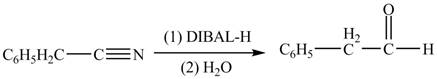
Figure 5
The product formed from the treatment of phenylacetonitrile
(f)
Interpretation: The product formed from the treatment of phenylacetonitrile
Concept introduction:
Answer to Problem 22.50P
The product formed from the treatment of phenylacetonitrile
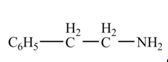
Explanation of Solution
Nitriles react with
![]()
Figure 6
The product formed from the treatment of phenylacetonitrile
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. Select to Edit Arrows H H Select to Add Arrows > H CFCI: Select to Edit Arrows H Select to Edit Arrowsarrow_forwardShow work with explanation needed. don't give Ai generated solutionarrow_forwardShow work. don't give Ai generated solutionarrow_forward
