
(a)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
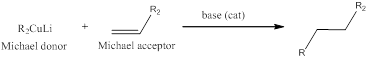
The formed enolate when treated with
(b)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
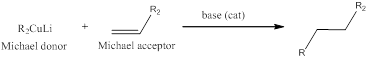
The formed enolate when treated with alkyl halide installs the alkyl group in the
Reduction of
(c)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
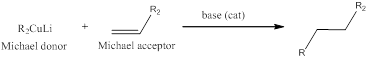
The formed enolate when treated with alkyl halide installs the alkyl group in the
Oxidation of the aldehyde with chromic acid gives the respective
(d)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Hydrolysis of acetal with aqueous acid leads to ketone.
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
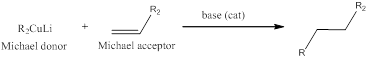
The formed enolate when treated with alkyl halide installs the alkyl group in the
When ketone is treated with ethylene glycol, the keto group is converted into an acetal.
(e)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Alcohol when treated with
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
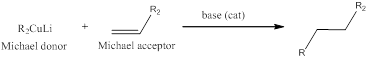
The formed enolate when treated with alkyl halide installs the alkyl group in the
Imine is formed when aldehyde is treated with a primary
(f)
Interpretation:
To identify the major product in the given set of reactions when treated with
Concept introduction:
Alcohol when treated with
The reaction between lithium dialkyl cuprate (Michael donor) which acts as a nucleophile for 1,4-conjugate addition to
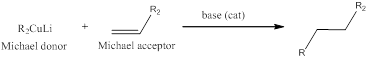
The formed enolate when treated with alkyl halide installs the alkyl group in the
Clemmenson reduction of aldehyde gives
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
ORGANIC CHEMISTRY-PRINT COMPANION (LL)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





