
Concept explainers
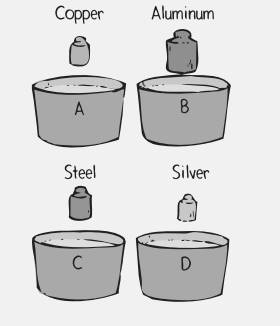
Four plastic-foam soup bowls contain the same amount of water at
Rank the bowls according to the maximum temperature of the water after the cylinders are added.
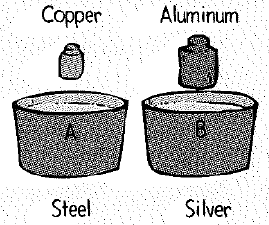
To rank: The bowls on the basis of maximum temperature of water after the cylinders are added.
Answer to Problem 22A
B> C> A> D.
Explanation of Solution
Introduction:
The specific heat capacity of a material is the quantity of heat required to raise the temperature of a unit mass of the material by 1 degree.
The given bowls are shown below.
Specific heat of all 5 substances in
Water-
Copper-
Aluminum-
Steel-
Silver-
As Aluminum has the highest specific heat, it will transfer heat more readily. Thus, it will have the highest temperature when dunking in the water. Steel will have second highest temperature and aluminium have third highest. While silver would have the lowest.
Conclusion:
Thus, according to the specific heat of all substances dunk in the water. Ranking of the maximum temperature of the water would be
Chapter 21 Solutions
Conceptual Physics: The High School Physics Program
Additional Science Textbook Solutions
The Cosmic Perspective Fundamentals (2nd Edition)
The Cosmic Perspective
University Physics (14th Edition)
Modern Physics
Introduction to Electrodynamics
Physics for Scientists and Engineers with Modern Physics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





