
Concept explainers
A vessel contains 1.00 × 104 oxygen molecules at 500 K. (a) Make an accurate graph of the Maxwell speed distribution function versus speed with points at speed intervals of 100 m/s. (b) Determine the most probable speed from this graph. (c) Calculate the average and rms speeds for the molecules and label these points on your graph. (d) From the graph, estimate the fraction of molecules with speeds in the range 300 m/s to 600 m/s.
(a)
The graph of the Maxwell speed distribution function versus speed with points at speed intervals of
Answer to Problem 21.62AP
The of the Maxwell speed distribution function versus speed with points at speed intervals of
Explanation of Solution
The Maxwell distribution curve is the graph between the distribution of speed and the change in speed or speed interval.
The number of molecules of oxygen in vessel is
Write the expression of Maxwell’s speed distribution function.
Here,
The mass of the molecules of oxygen
Here,
The molecular mass of the oxygen molecules in
Substitute
Substitute
Substitute the values of
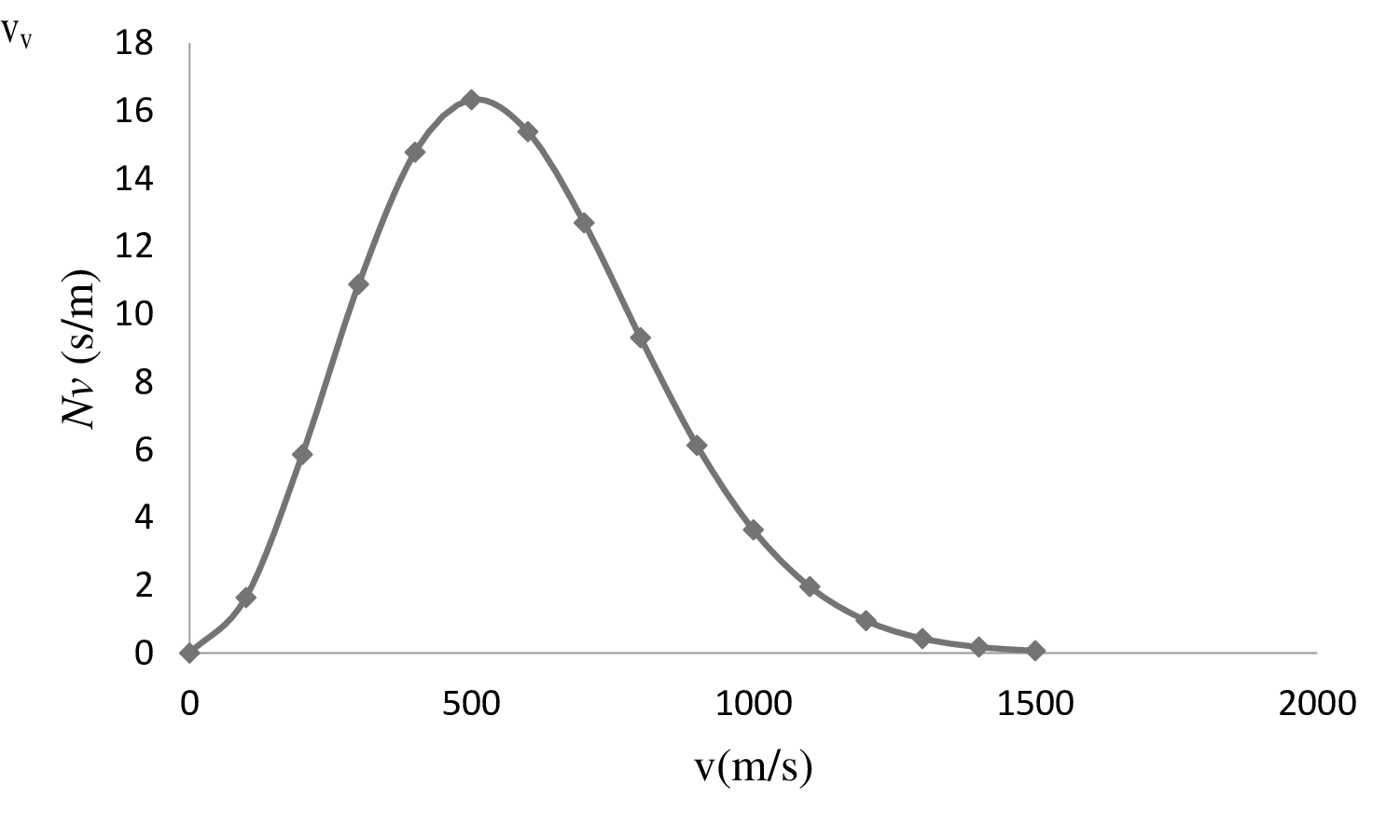
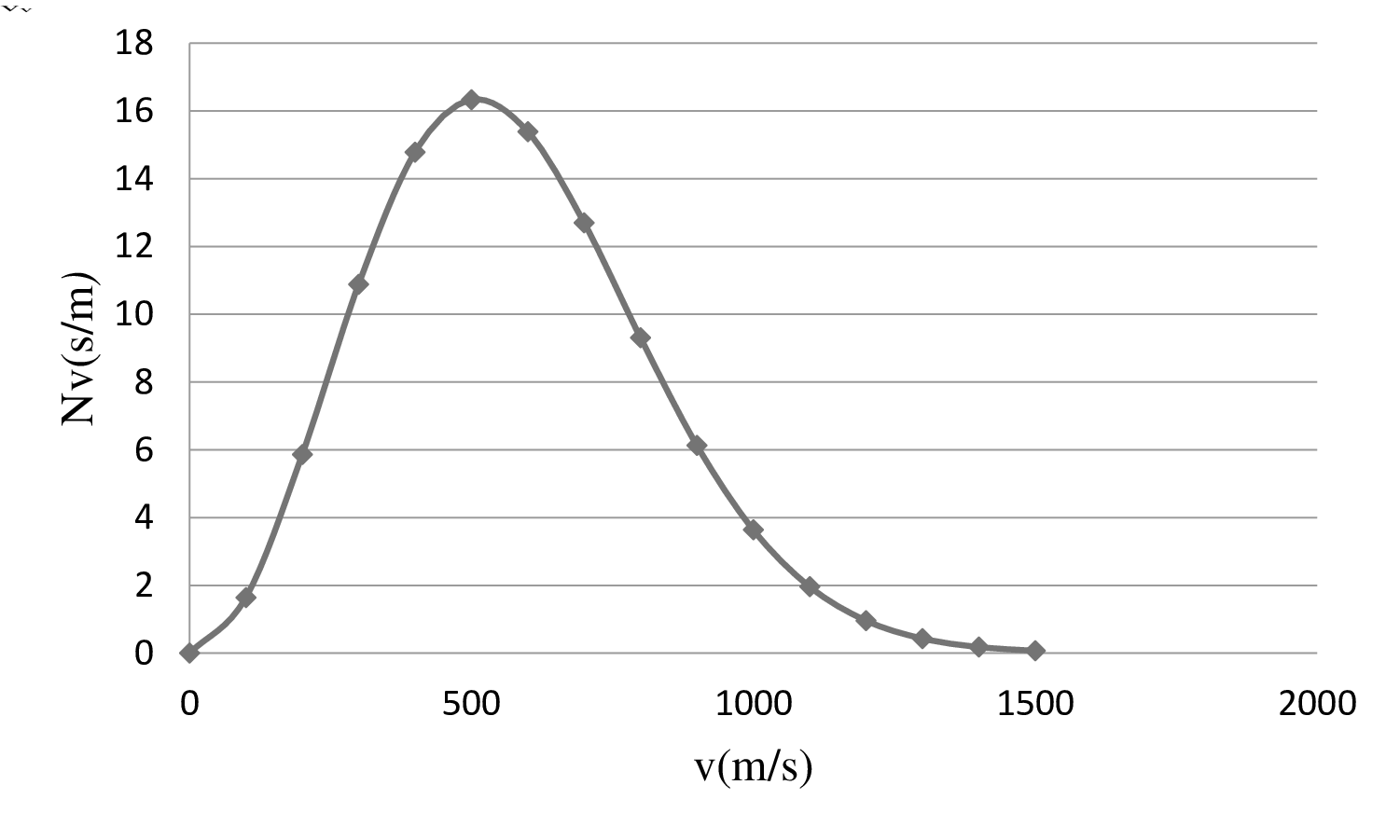
| 0 | 0 |
| 100 | 1.64 |
| 200 | 5.86 |
| 300 | 10.88 |
| 400 | 14.78 |
| 500 | 16.33 |
| 600 | 15.39 |
| 700 | 12.7 |
| 800 | 9.31 |
| 900 | 6.13 |
| 1000 | 3.64 |
| 1100 | 1.961 |
| 1200 | 0.96 |
| 1300 | 0.43 |
| 1400 | 0.18 |
| 1500 | 0.07 |
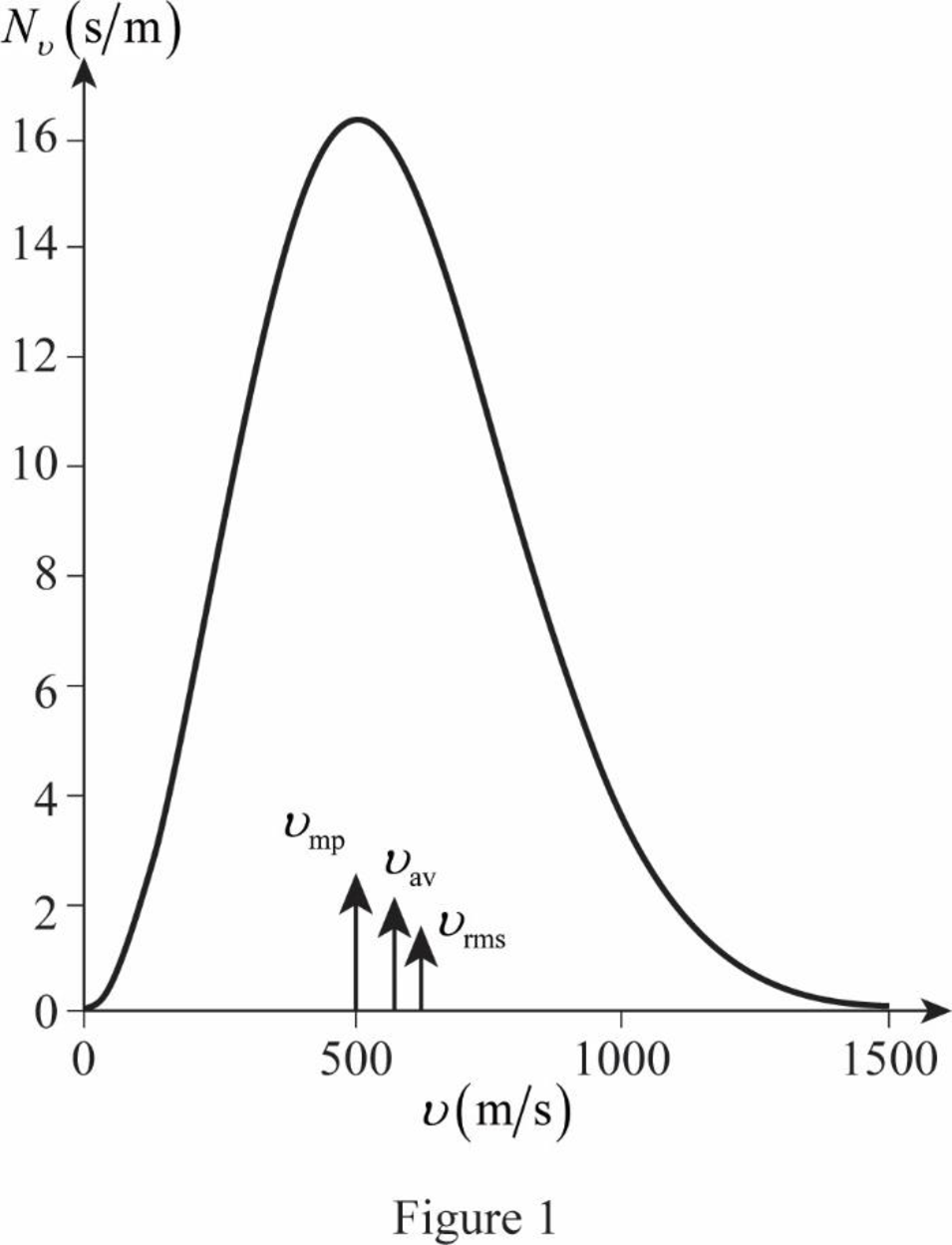
On the basis of the table, a graph is plotted below;
(b)
The most probable speed from the graph.
Answer to Problem 21.62AP
The most probable speed is
Explanation of Solution
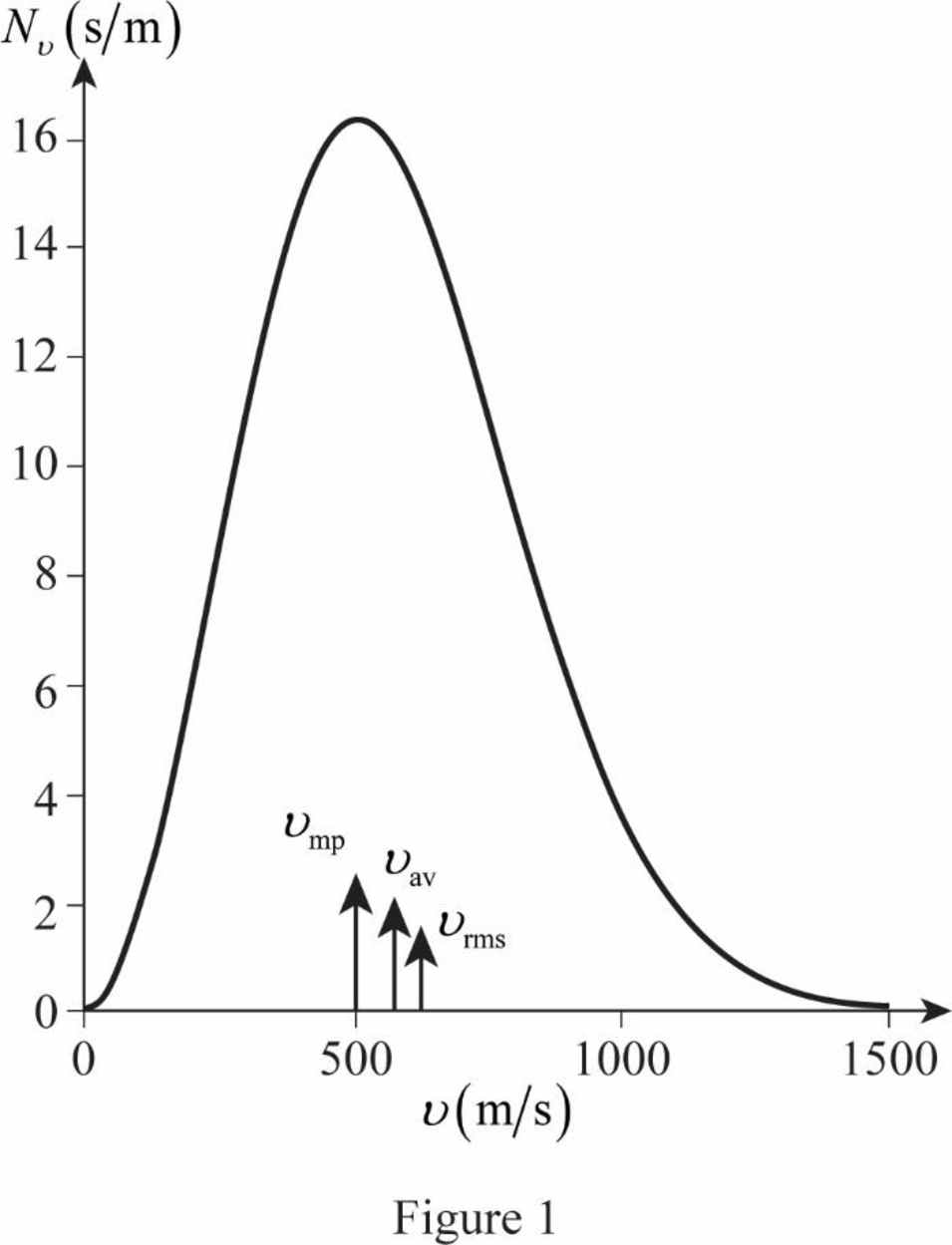
The most probable speed occurs where
Conclusion:
Therefore, the most probable speed is
(c)
The average and rms speeds for the molecules and label these points on the graph.
Answer to Problem 21.62AP
The average and rms speeds for the molecules is
Explanation of Solution
Write the expression of average velocity.
The mass of the molecules of oxygen
Substitute
The molecular mass of the oxygen molecules in
Substitute
Thus, the average speed is
Write the expression of rms velocity.
Substitute
Substitute
Thus, the rms velocity of the oxygen molecules is
The graph of Maxwell’s curve is shown below;
The point
Conclusion:
Therefore, the average and rms speeds for the molecules is
(d)
The fraction of molecules with the speed in the range of
Answer to Problem 21.62AP
The fraction of molecules with the speed in the range of
Explanation of Solution
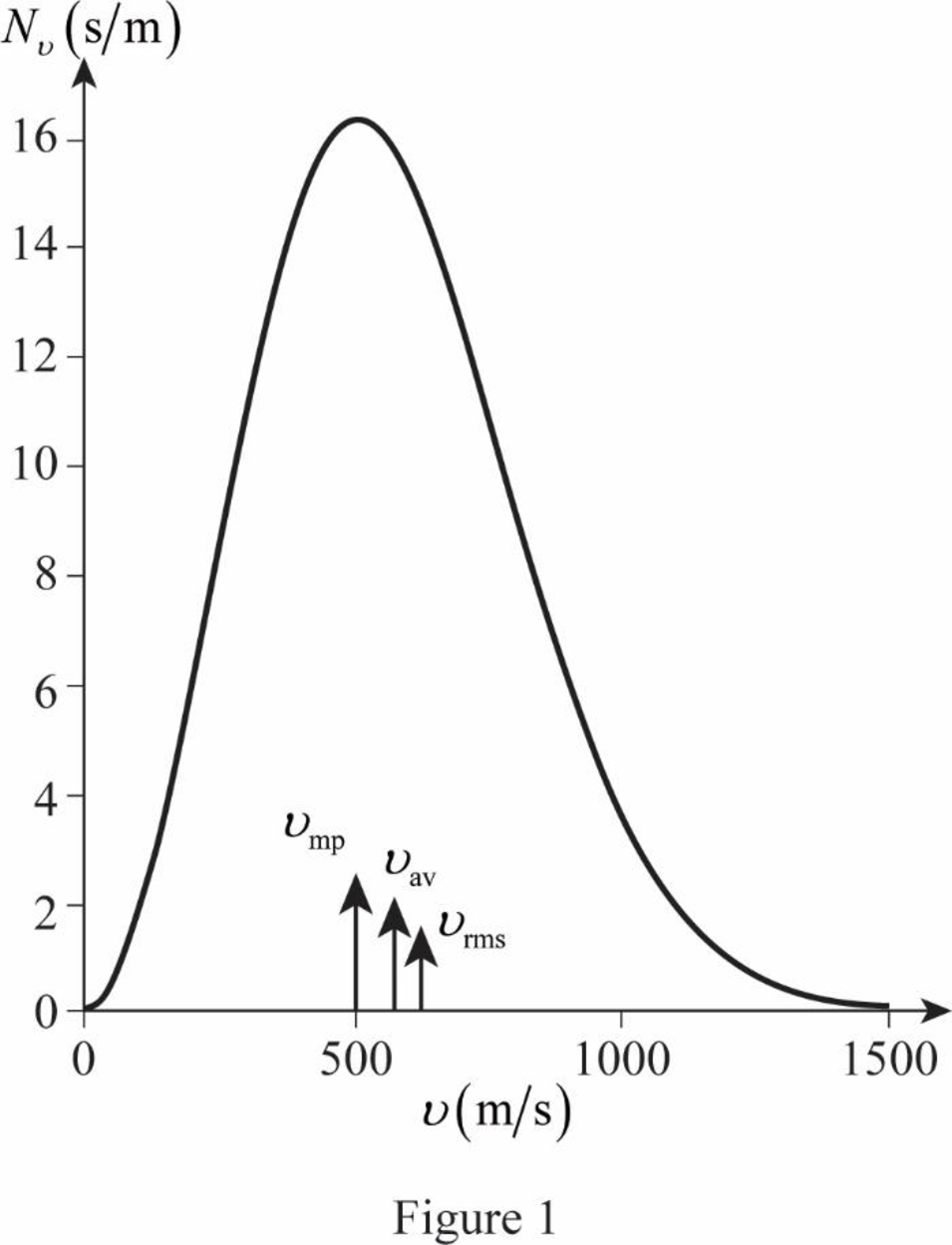
The figure given below shows the Maxwell’s curve,
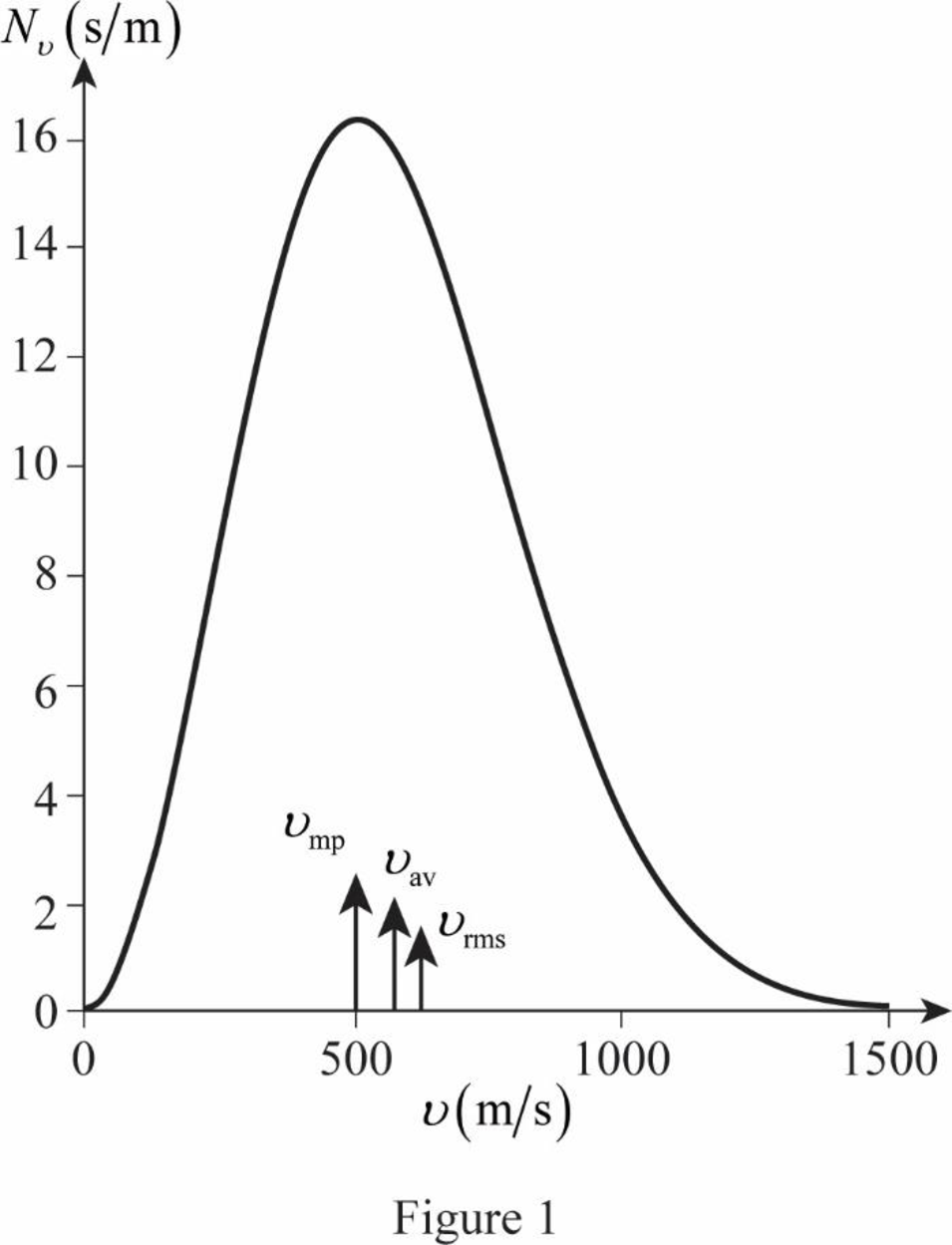
The area under the distribution curve in the range
Conclusion:
Write the area under the curve in the range
Therefore, the fraction of molecules with the speed in the range of
Want to see more full solutions like this?
Chapter 21 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
- An ideal gas is contained in a vessel at 300 K. The temperature of the gas is then increased to 900 K. (i) By what factor does the average kinetic energy of the molecules change, (a) a factor of 9, (b) a factor of 3, (c) a factor of 3, (d) a factor of 1, or (e) a factor of 13? Using the same choices as in part (i), by what factor does each of the following change: (ii) the rms molecular speed of the molecules, (iii) the average momentum change that one molecule undergoes in a collision with one particular wall, (iv) the rate of collisions of molecules with walls, and (v) the pressure of the gas?arrow_forwardFind (a) the most probable speed, (b) the average speed, and (c) the rms speed for nitrogen molecules at 295 K.arrow_forwardCylinder A contains oxygen (O2) gas, and cylinder B contains nitrogen (N2) gas. If the molecules in the two cylinders have the same rms speeds, which of the following statements is false? (a) The two gases haw different temperatures. (b) The temperature of cylinder B is less than the temperature of cylinder A. (c) The temperature of cylinder B is greater than the temperature of cylinder A. (d) The average kinetic energy of the nitrogen molecules is less than the average kinetic energy of the oxygen molecules.arrow_forward
- Consider a gas filling two connected chambers that are separated by a removable barrier (Fig. P20.68). The gas molecules on the left (red) are initially at a higher temperature than the ones on the right (blue). When the barrier between the two chambers is removed, the molecules begin to mix and move from one chamber to the other. a. Describe what happens to the temperature in the left chamber and in the right chamber as time goes on, once the barrier is open. Discuss in terms of the mixing of the molecules from each gas. b. Describe what happens to the most probable speed and average speed in the left chamber and in the right chamber as time goes on, once the barrier is open. Do they increase or decrease by the same factor? Explain. FIGURE P20.68 Problems 68 and 69.arrow_forwardOn a hot summer day, the density of air at atmospheric pressure at 35.0C is 1.1455 kg/m3. a. What is the number of moles contained in 1.00 m3 of an ideal gas at this temperature and pressure? b. Avogadros number of air molecules has a mass of 2.85 102 kg. What is the mass of 1.00 m3 of air? c. Does the value calculated in part (b) agree with the stated density of air at this temperature?arrow_forwardOne process for decaffeinating coffee uses carbon dioxide ( M=44.0 g/mol) at a molar density of about 14,0 mol/m3 and a temperature of about 60 . (a) Is CO2 a solid, liquid, gas, or supercritical fluid under those conditions? (b) The van der Waals constants for carbon dioxide are a=0.3658 Pa m6/mol2 and b=4.286105 m3/mol. Using the van der Waals equation, estimate pressure of CO2 at that temperature and density. `arrow_forward
- In the text, it was shown that N/V=2.681025m3 for gas at STP. (a) Show that this quantity is equivalent to N/V=2.681019cm3, as stated. (b) About how many atoms are mere in one m3 (a cubic micrometer) at STP? (c) What does your answer to part (b) imply about the separation of Mama and molecules?arrow_forwardFrom the MaxwellBoltzmann speed distribution, show that the most probable speed of a gas molecule is given by Equation 16.23. Note: The most probable speed corresponds to the point at which the slope of the speed distribution curve dNv/dv is zero.arrow_forwardDecades ago, it was thought that huge herbivorous dinosaurs such as Apatosaurus and Brachiosaurus habitually walked on the bottom of lakes, extending their long necks up to the surface to breathe. Brarhiosaurus had its nostrils on the top of its head. In 1977, Knut Schmidt-Nielsen pointed out that breathing would be too much work for such a creature. For a simple model, consider a sample consisting of 10.0 L of air at absolute pressure 2.00 atm, with density 2.40 kg/m3, located at the surface of a freshwater lake. Find the work required to transport it to a depth of 10.3 m, with its temperature, volume, and pressure remaining constant. This energy investment is greater than the energy that can be obtained by metabolism of food with the oxygen in that quantity of air.arrow_forward
- Fifteen identical particles have various speeds: one has a speed of 2.00 m/s, two have speeds of 3.00 m/s, three have speeds of 5.00 m/s, four have speeds of 7.00 m/s, three have speeds of 9.00 m/s, and two have speeds of 12.0 m/s. Find (a) the average speed, (b) the rms speed, and (c) the most probable speed of these particles.arrow_forwardUsing a numerical integration method such as Simpson's rule, find the fraction of molecules in a sample of oxygen gas at a temperature of 250 K that have speeds between 100 m/s and 150 m/s. The molar mass of oxygen (O2) is 32.0 g/mol. A precision to two significant digits is enough.arrow_forwardOne cylinder contains helium gas and another contains krypton gas at the same temperature. Mark each of these statements true, false, or impossible to determine from the given information. (a) The rms speeds of atoms in the two gases are the same. (b) The average kinetic energies of atoms in the two gases are the same. (c) The internal energies of 1 mole of gas in each cylinder are the same. (d) The pressures in the two cylinders ale the same.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning





