
Concept explainers
(a)
Interpretation: The structure corresponding to the given IUPAC name is to be stated.
Concept introduction: One should follow the given steps to derive the structure of an
Answer to Problem 21.4P
The structure corresponding to the given IUPAC name is,
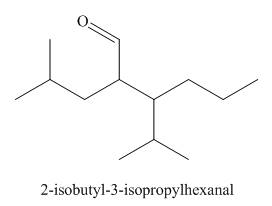
Explanation of Solution
The IUPAC name of the given compound is
One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given IUPAC name suggests the presence of
Thus, the structure corresponding to the given IUPAC name is,

Figure 1
The structure corresponding to the given IUPAC name is shown in Figure 1.
(b)
Interpretation: The structure corresponding to the given IUPAC name is to be stated.
Concept introduction: One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.4P
The structure corresponding to the given IUPAC name is,
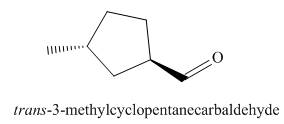
Explanation of Solution
The IUPAC name of the given compound is
One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given IUPAC name suggests the presence of

Figure 2
The structure corresponding to the given IUPAC name is shown in Figure 2.
(c)
Interpretation: The structure corresponding to the given IUPAC name is to be stated.
Concept introduction: One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.4P
The structure corresponding to the given IUPAC name is,
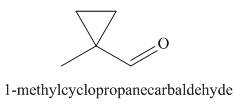
Explanation of Solution
The IUPAC name of the given compound is
One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given IUPAC name suggests the presence of

Figure 3
The structure corresponding to the given IUPAC name is shown in Figure 3.
(d)
Interpretation: The structure corresponding to the given IUPAC name is to be stated.
Concept introduction: One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
Answer to Problem 21.4P
The structure corresponding to the given IUPAC name is,
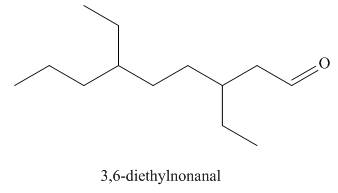
Explanation of Solution
The IUPAC name of the given compound is
One should follow the given steps to derive the structure of an aldehyde from its IUPAC name. The first step is finding of longest parent chain that contains an aldehyde group. The second step is changing of -e ending of the parent alkane to the suffix -al. However, when an aldehyde
The given IUPAC name suggests the presence of
Thus, the structure corresponding to the given IUPAC name is,

Figure 4
The structure corresponding to the given IUPAC name is shown in Figure 4.
Want to see more full solutions like this?
Chapter 21 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Give the structure corresponding to each IUPAC name.a. 2-isobutyl-3-isopropylhexanalb. trans-3-methylcyclopentanecarbaldehydec. 1-methylcyclopropanecarbaldehyded. 3,6-diethylnonanalarrow_forward17. CH2CH3 The correct name for the compound given above is which of the follow ing? a. 1-propyl-3-ethy-4-methylbenzene b. 4-cyclopropy-2-ethyl-1-methy lbenzene c. p.o - methyl, ethyleyclopropylbenzene d. 1-propyl-3-ethyl-4-methyleyclohexane 18. The correct structure for 1,4-dicyc lopropyleyc lohexane is which of the following? a. CH3-CH2-CH2- b. CH2 CH2-CH3 с. CH,-CH2-CH2 CH-CH, CH d.arrow_forwardGive the IUPAC name for each compound. CH3 CH2CH3 Br a. PHCH(CH3)2 b. С. d.arrow_forward
- Give the structure corresponding to each IUPAC name. a. 3-methyl-3-pentanold. 1,3-propanediol b. 4-methyl-2-pentanole. 3,5-dimethylcyclohexanol c. 2,4-dimethyl-2-hexanolf. 6,6-diethyl-4-nonanolarrow_forwardDraw the structure corresponding to each IUPAC name. a.3-ethyl-2-methylhexane b. sec-butylcyclopentane c.4-isopropyl-2,4,5-trimethylundecane d.cyclobutylcycloheptane e.3-ethyl-1,1-dimethylcyclohexane f. 4-butyl-1,1-diethylcyclooctane g.6-isopropyl-2,3-dimethyldodecane h. 2,2,6,6,7-pentamethyloctane i. cis-1-ethyl-3-methylcyclopentane j. trans-1-tert-butyl-4-ethylcyclohexanearrow_forwardWhat is the IUPAC name of the following compound? Br CH3 H3C O A. 5-Bromo-2-methylphenyl ethanoate B. 3-Bromo-6-methylphenyl ethanoate C. 4-Bromo-2-{oxy-(1-oxoethyl)} toluene EO D. Methyl-5-bromo-2-methyl benzoatearrow_forward
- What is the IUPAC name for the following compound? CH₂CH₂ C CH3-CH₂-CH-CH₂-CH-CH-CH-CH₂ CI CH3 Select one: O A. 3,7-dichloro-5-ethyl-6-methyloctane O B. 2-chloro-3-methyl-4-(2-chlorobutyl) hexane O C. 2,6-dichloro-4-ethyl-3-methyloctane O D. 3-chloro-5-(2-chloro-1-methylpropyl) heptane OE. 2,3,4,6-dichloroethylmethyoctanearrow_forwardWhat is the IUPAC name of the following compound? OH Br N=C O A. 3-bromo-4-hydroxybenzonitrile B. 2-bromo-4-cyanophenol C. 2-bromo-4-cyano-1-hydroxybenzene D. 2-bromo-4-cyanobenzanolarrow_forwardMatch the letter corresponding to each structure to its proper functional group name. A. H,C CH В. H.C CH, C. HC-C-OH Ketone [ Choose ] [Choose] Alkene Carboxylic Acid Ketone Aldehydearrow_forward
- 6. Draw the following compounds. a. 2-pentanol b. ethylpropylamine c. methyl propyl ether d. butyl propyl ketone abayoqtheo ghidollo WHO H3--3 HO HO HO HO HOarrow_forwardH3C. H3C CH3 Br 1-propanolarrow_forwardD. Draw a structural formula for these compounds. 1. 1-Chloro-2-propanone 2. 3-Hydroxybutanal 3. 4-Hydroxy-4-methyl-2-pentanone 4. 3-Methyl-3-phenylbutanal 5. 1,3-Cyclohexandionearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





