
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 21.3P
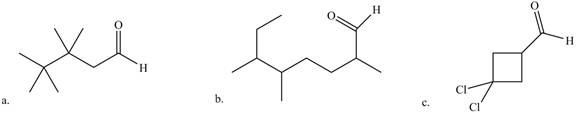
Give the IUPAC name for each
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please correct answer and don't used hand raiting don't used Ai solution
Please correct answer and don't used hand raiting
Please correct answer and don't used hand raiting
Chapter 21 Solutions
Organic Chemistry
Ch. 21 - Rank the following compounds in order of...Ch. 21 - Prob. 21.2PCh. 21 - Give the IUPAC name for each aldehyde.Ch. 21 - Prob. 21.4PCh. 21 - Give the IUPAC name for each ketone.Ch. 21 - Prob. 21.6PCh. 21 - Prob. 21.7PCh. 21 - The boiling point of is significantly higher than...Ch. 21 - Which carbonyl group in each pair absorbs at a...Ch. 21 - Problem 21.10 Draw the structure of all...
Ch. 21 - Prob. 21.11PCh. 21 - Prob. 21.12PCh. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Problem 21.15 Draw the product of each...Ch. 21 - Prob. 21.16PCh. 21 - Problem 21.17 Draw the products of the following...Ch. 21 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 21 - Problem 21.19 Draw the products (including...Ch. 21 - Problem 21.20 What starting materials are needed...Ch. 21 - Prob. 21.21PCh. 21 - Problem 21.22 The product formed when reacts with...Ch. 21 - Prob. 21.23PCh. 21 - Prob. 21.24PCh. 21 - Prob. 21.25PCh. 21 - Prob. 21.26PCh. 21 - Prob. 21.27PCh. 21 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.29 Draw the products of each...Ch. 21 - Problem 21.30 Label each compound as an acetal, a...Ch. 21 - Problem 21.31 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.32 Draw the products of each...Ch. 21 - Problem 21.33 Safrole is a naturally occurring...Ch. 21 - Prob. 21.34PCh. 21 - Problem 21.35 How would you use a protecting group...Ch. 21 - Prob. 21.36PCh. 21 - Problem 21.37 Two naturally occurring compounds...Ch. 21 - Problem 21.38 Draw the products of each...Ch. 21 - Prob. 21.39PCh. 21 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 21 - 21.41 Rank the following compounds in order of...Ch. 21 - Prob. 21.42PCh. 21 - 21.43 Give the IUPAC name for each compound.
a....Ch. 21 - 21.44 Give the structure corresponding to each...Ch. 21 - Prob. 21.45PCh. 21 - 21.46 Draw the products of each reaction.
a. e....Ch. 21 - Prob. 21.47PCh. 21 - 21.48 Draw all stereoisomers formed in each...Ch. 21 - Prob. 21.49PCh. 21 - What products are formed when each acetal is...Ch. 21 - Prob. 21.51PCh. 21 - Prob. 21.52PCh. 21 - Which compound forms the higher concentration of...Ch. 21 - Prob. 21.54PCh. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Devise a synthesis of each alkene using a Wittig...Ch. 21 - Devise a synthesis of each compound from...Ch. 21 - Prob. 21.60PCh. 21 - Devise a synthesis of each compound from ethanol...Ch. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - 21.64 Draw a stepwise mechanism for the following...Ch. 21 - 21.65 Draw a stepwise mechanism f or the following...Ch. 21 - Prob. 21.66PCh. 21 - 21.67 Draw a stepwise mechanism for each...Ch. 21 - 21.68 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.69PCh. 21 - Prob. 21.70PCh. 21 - Prob. 21.71PCh. 21 - Prob. 21.72PCh. 21 - 21.73 Although the carbonyl absorption of cyclic...Ch. 21 - 21.74 Use the and data to determine the...Ch. 21 - 21.75 A solution of acetone in ethanol in the...Ch. 21 - Compounds A and B have molecular formula ....Ch. 21 - 21.77 An unknown compound C of molecular formula ...Ch. 21 - 21.78 An unknown compound D exhibits a strong...Ch. 21 - Prob. 21.79PCh. 21 - -D-Glucose, a hemiacetal, can be converted to a...Ch. 21 - 21.81 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.82PCh. 21 - 21.83 Draw a stepwise mechanism f or the...Ch. 21 - Prob. 21.84PCh. 21 - Prob. 21.85PCh. 21 - 21.86 Draw stepwise mechanism for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY