
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 20, Problem 21P
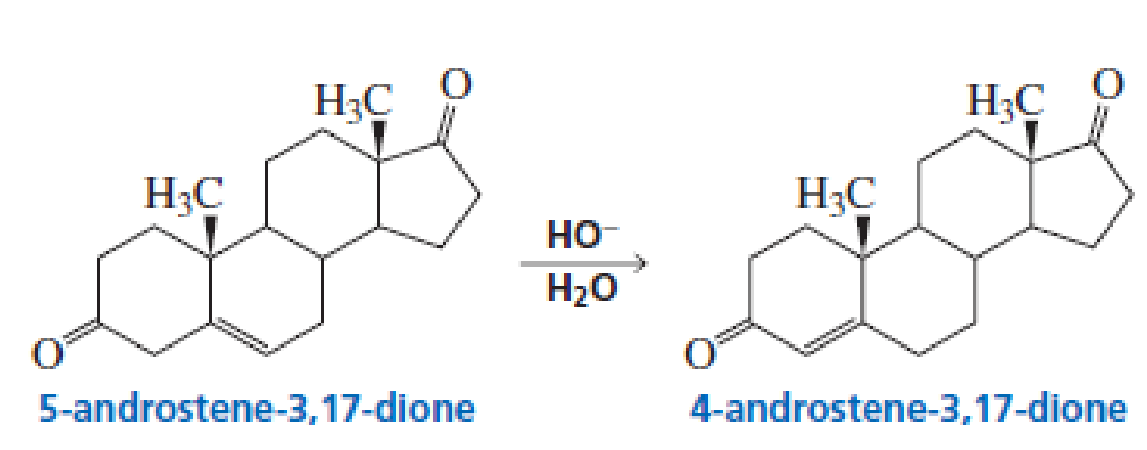
5-Androstene-3,17-dione is isomerized to 4-androstene-3,17-dione by hydroxide ion. Propose a mechanism for this reaction.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
5-Androstene-3,17-dione is isomerized to 4-androstene-3,17-dione by hydroxide ion. Propose a mechanism for this reaction
Acid-catalyzed dehydration of 3-methyl-2-butanol gives three alkenes: 2-methyl-2- butene, 3-methyl-1-butene, and 2-methyl-1-butene. Propose a mechanism to account for the formation of each product
Benzene + nitration, followed by bromination, then reduction of the nitro group to an amine, followed by diazonization, addition of copper I
cyanide to the reaction and the product is
O benzoyl nitrile
O ortho-bromoaniline
O para-bromo-nitrobenzene
O 3,5-dibromnobenzonitrile
O para-bromobenzyl bromide
Chapter 20 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 20.1 - Prob. 1PCh. 20.2 - Prob. 2PCh. 20.2 - Prob. 3PCh. 20.2 - Draw the structure of an optically active fat...Ch. 20.4 - Prob. 6PCh. 20.4 - Prob. 7PCh. 20.4 - The membrane phospholipids in deer have a higher...Ch. 20.4 - Prob. 9PCh. 20.6 - Prob. 10PCh. 20.6 - Prob. 11P
Ch. 20.6 - Prob. 12PCh. 20.7 - Propose a mechanism for the biosynthesis of...Ch. 20.7 - Prob. 14PCh. 20.8 - Draw the individual 1,2-hydride and 1,2-methyl...Ch. 20.9 - Prob. 16PCh. 20 - Prob. 17PCh. 20 - Prob. 18PCh. 20 - Cardiolipins are found in heart muscles. Draw the...Ch. 20 - Prob. 20PCh. 20 - 5-Androstene-3,17-dione is isomerized to...Ch. 20 - Prob. 22PCh. 20 - Prob. 23PCh. 20 - Prob. 24PCh. 20 - Eudesmol is a sesquiterpene found in eucalyptus....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When 4-hydroxybutanoic acid is treated with an acid catalyst, it forms a lactone (a cyclic ester). Draw the structural formula of this lactone and propose a mechanism for its formationarrow_forward1,4-Dioxane is made commercially by the acid-catalyzed condensation of an alcohol. Propose a mechanism for this reaction.arrow_forwardBisphenol A is made on a large scale by a condensation of phenol with acetone. Suggest an appropriate catalyst, and propose a mechanism for this reaction.arrow_forward
- Aldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forwardEthylene oxide is the starting material for the synthesis of 1,4-dioxane. Propose a mechanism for each step in this synthesis.arrow_forwardTreatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forward
- One frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardDihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesul- fonic acid, ARSO,H. Propose a mechanism for this conversion. ARSO,H OH + H,O Tetrahydrofurfuryl Dihydropyran alcoholarrow_forwardWrite the mechanisms for the following reactions: Decanoic acid + Ethyl alcohol Propanoic acid + Ethyl alcohol Salicylic acid + Benzyl alcohol Decanoic acid + 3-methyl-1-butanolarrow_forward
- Propose a mechanism for the acid-catalyzed hydration of methylidenecyclohexane to give 1-methylcyclohexanol. Which step in your mechanism is rate-determining?arrow_forwardPropose a mechanism for the acid-catalyzed hydration of 1-methylcyclohexene to give 1-methylcyclohexanol. Which step in your mechanism is rate-determining?arrow_forward1 The reaction of an a-diketone with concentrated sodium or potassium hydroxide to give the salt of an a-hydroxyacid is given the general name benzil-benzilic acid rearrangement. It is illustrated by the conversion of benzil to sodium benzilate and then to benzilic acid. Propose a mechanism for this rearrangement. O O НО О Но о H,O Ph—С—С—Рh + NaOH 7 Ph —С—С—O Nat HCI Ph—С—С—ОН H,O Ph Ph Benzil Sodium benzilate Benzilic acid (an a-diketone)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY